
In this application note, we demonstrated some challenging chiral separations of pyrethroids with varying stereochemical complexity using a Waters ACQUITY UPC2 System.
Pyrethroids are synthetic compounds similar to natural pyrethrins. They are commonly used in both commercial agriculture and households as insecticides and insect repellents. Unlike other common classes of insecticides, such as organophosphates and carbamates that are acetylcholine esterase inhibitors or nicotinic acetycholine agonists, the pyrethroids act upon neuronal sodium channels for their insecticidal effect. Due to metabolic rate differences between species, many pyrethroids possess moderate to good selectivity for insect versus mammalian biology, allowing for their relatively safe application.1
Many pyrethroids possess multiple stereogenic centers, as shown in Figure 1. Individual stereoisomers can have vastly different biological activities. Furthermore, environmental degradation of many insecticides is enzymatic and sensitive to the stereochemical configuration. As a result, rapid, reliable, and precise determination of the isomeric ratio of these chiral pesticides is of great importance not only for product formulation, but also for the subsequent study of the pharmacokinetics, metabolism, and the environmental fates of the individual pesticide isomers.
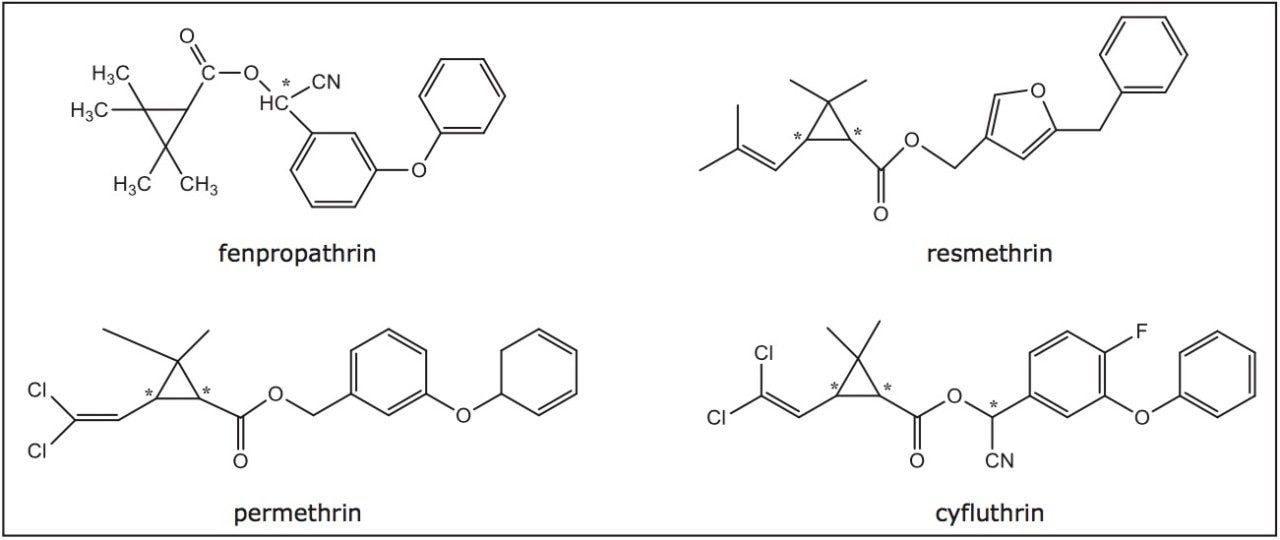
Historically, chiral separations of pyrethroids have primarily been carried out using HPLC on chiral stationary phases (CSPs), capillary electrophoresis (CE), and gas chromatography (GC).1 More recently, supercritical fluid chromatography (SFC) has been applied to these separations as well, often resulting in improved resolution and reduced run time. In this application note, we present the enantiomeric and diastereomeric separations of four pyrethroids with varying stereochemical complexity using a Waters ACQUITY UPC2 System.
The pesticide samples used in this study were purchased from Reidel-de Haen, Pestanal, Sigma and Fluka, with their structures shown in Figure 1. All samples were dissolved in isopropanol (IPA) for analyses.
|
UPC2 conditions |
|
|---|---|
|
System: |
ACQUITY UPC2 |
|
Detection: |
PDA |
|
Columns: |
CHRALCEL OJ-H and CHIRALPAK IC (4.6 x 150 mm, 5 μm) obtained from Chiral Technologies |
Empower 3 Software
Other key experimental parameters are listed in the respective figure captions.
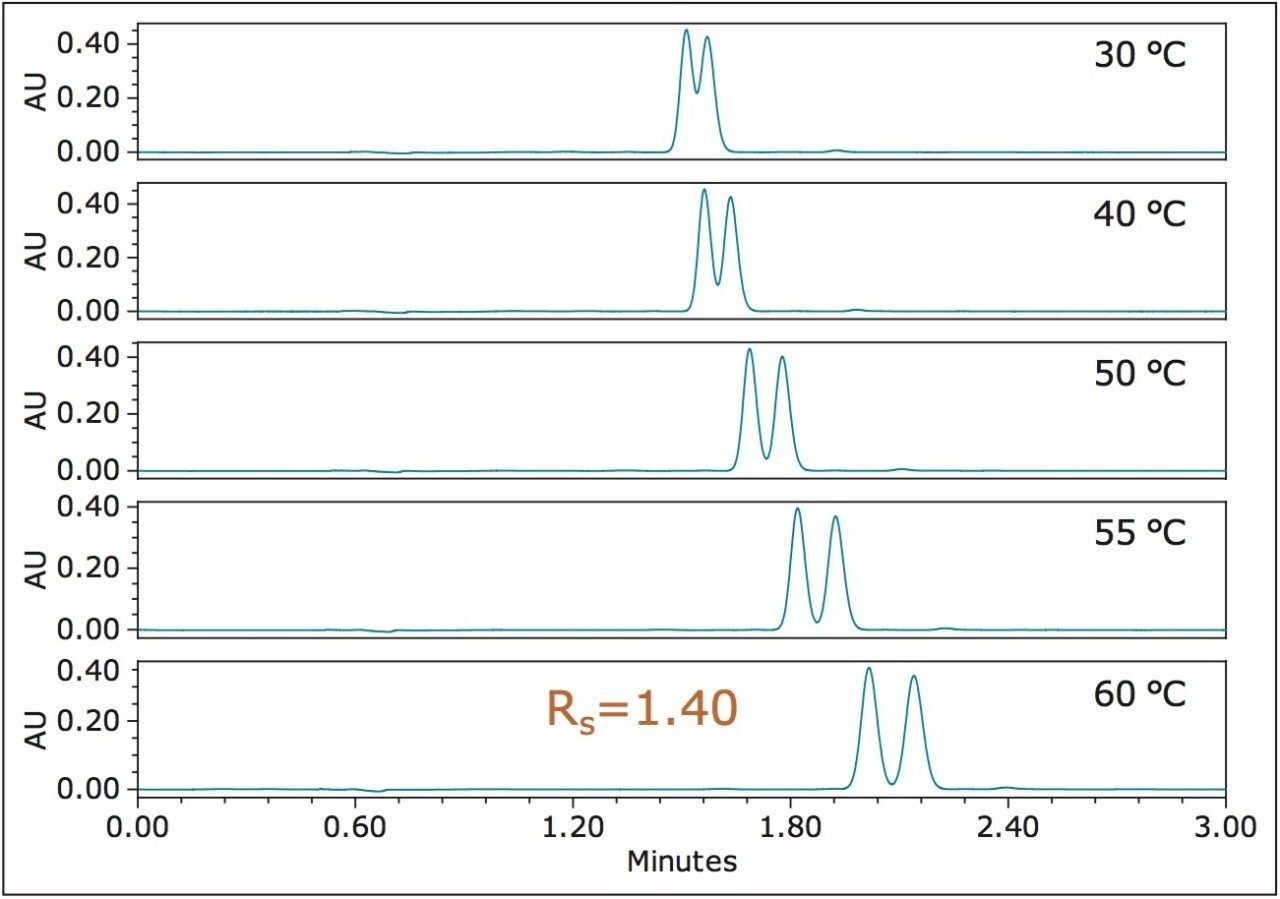
Chiral method development often starts with screening different stationary phases and co-solvent/additives. After extensive screening of fenpropathrin, an OJ-H column and methanol were selected for further optimization. Figure 2 shows the UPC2 chromatograms of fenpropathrin at different temperatures. As the temperature increased, the solvation strength of supercritical CO2 decreased and retention time became longer. This allowed for more interactions between analytes and the chiral selectors on stationary phases, resulting in higher resolution. While temperature is often perceived as a secondary parameter for selectivity adjustment in achiral SFC, the current example clearly demonstrates that temperature could dramatically alter the chiral resolution.
To further improve the resolution, the flow rate was lowered to 1.5 mL/min while other parameters remained the same, and the resulting chromatogram is shown in Figure 3. A baseline resolution (Rs=1.67) was achieved. Contrary to typical reverse phase or normal phase liquid chromatography for an achiral separation where a dominant interaction usually dictates retention, chiral chromatographywith SFC typically results from multiple interactions between analytes and stationary phases. These interactions could have different dependence on typical experimental parameters, including flow rate, pressure, and temperature. It is, therefore, important to systematically explore all possible contributors to achieve maximum resolution.

Figure 4 shows the UPC2 separation of permethrin, a pyrethroid with two chiral centers. The four isomers were baseline resolved in less than 4 min on an OJ-H column (4.6 x 150 mm, 5 μm). A 100-min GC separation
resolved three out of the four isomers.2 The four isomers couldn’t be completely separated by either reverse phase or normal phase LC.3
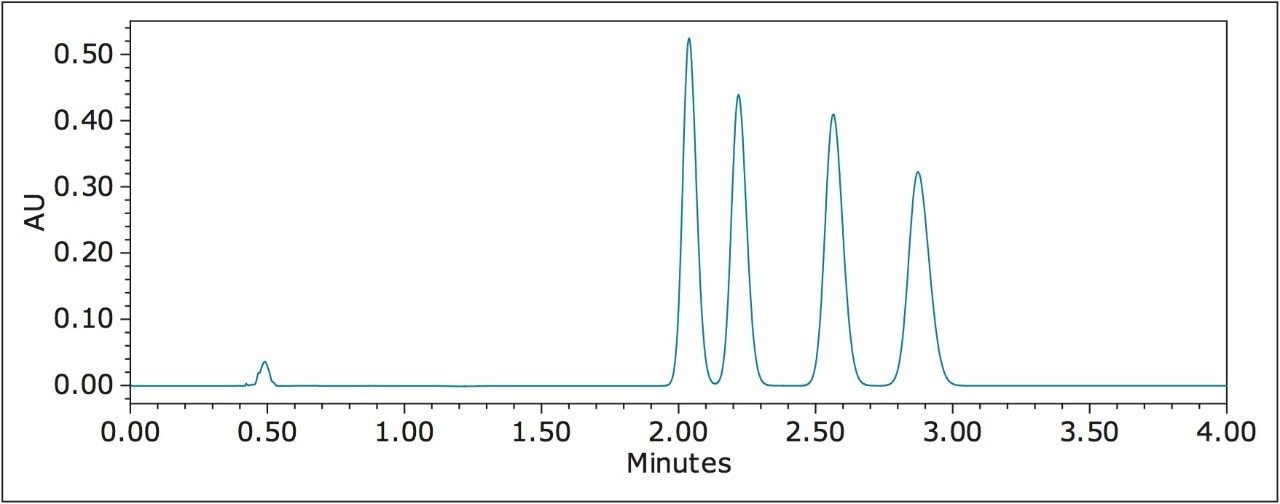
Figure 5 shows a UPC2 chromatogram of resmethrin, another pyrethroid with two chiral centers. Despite the structural similarity between permethrin and resmethrin, the baseline resolution of all four isomers required two OJ-H columns coupled in tandem, and a reduced flow rate. Increasing column length is one of the most fundamental and facile means to improve chromatographic resolution. Column coupling has been used for both analytical and preparative SFC,4-5 when selectivity or retentivity was inadequate to improve resolution. Compared with HPLC, SFC holds the following unique advantage: the viscosity of liquid CO2 under typical SFC conditions is 10- to 100-fold lower than those commonly used HPLC solvents. The low viscosity of liquid CO2 allows for column coupling, hence, extending column length for higher chromatographic efficiency without generating a prohibitive pressure drop across columns.
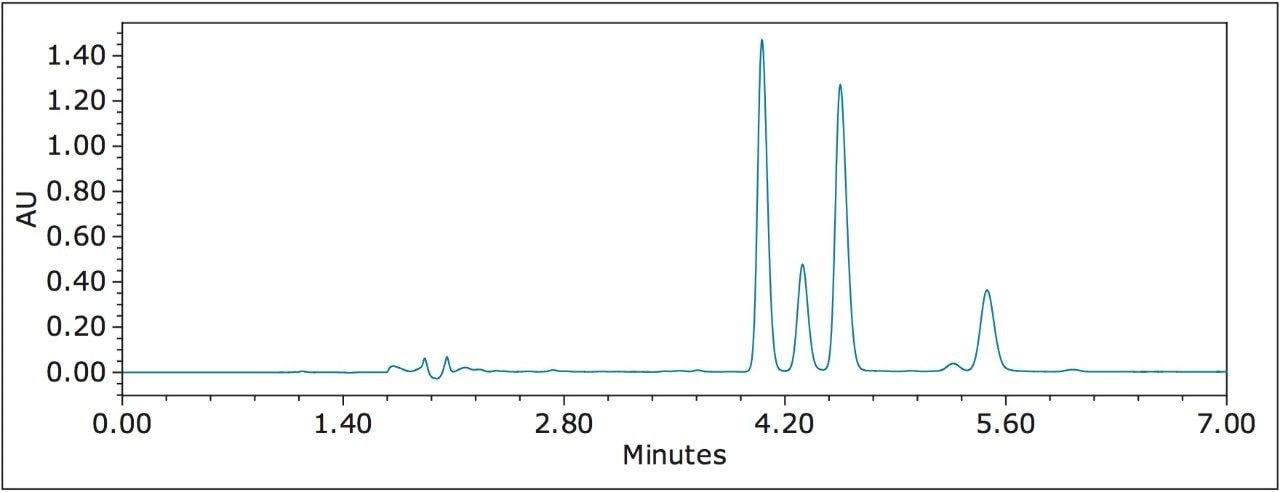
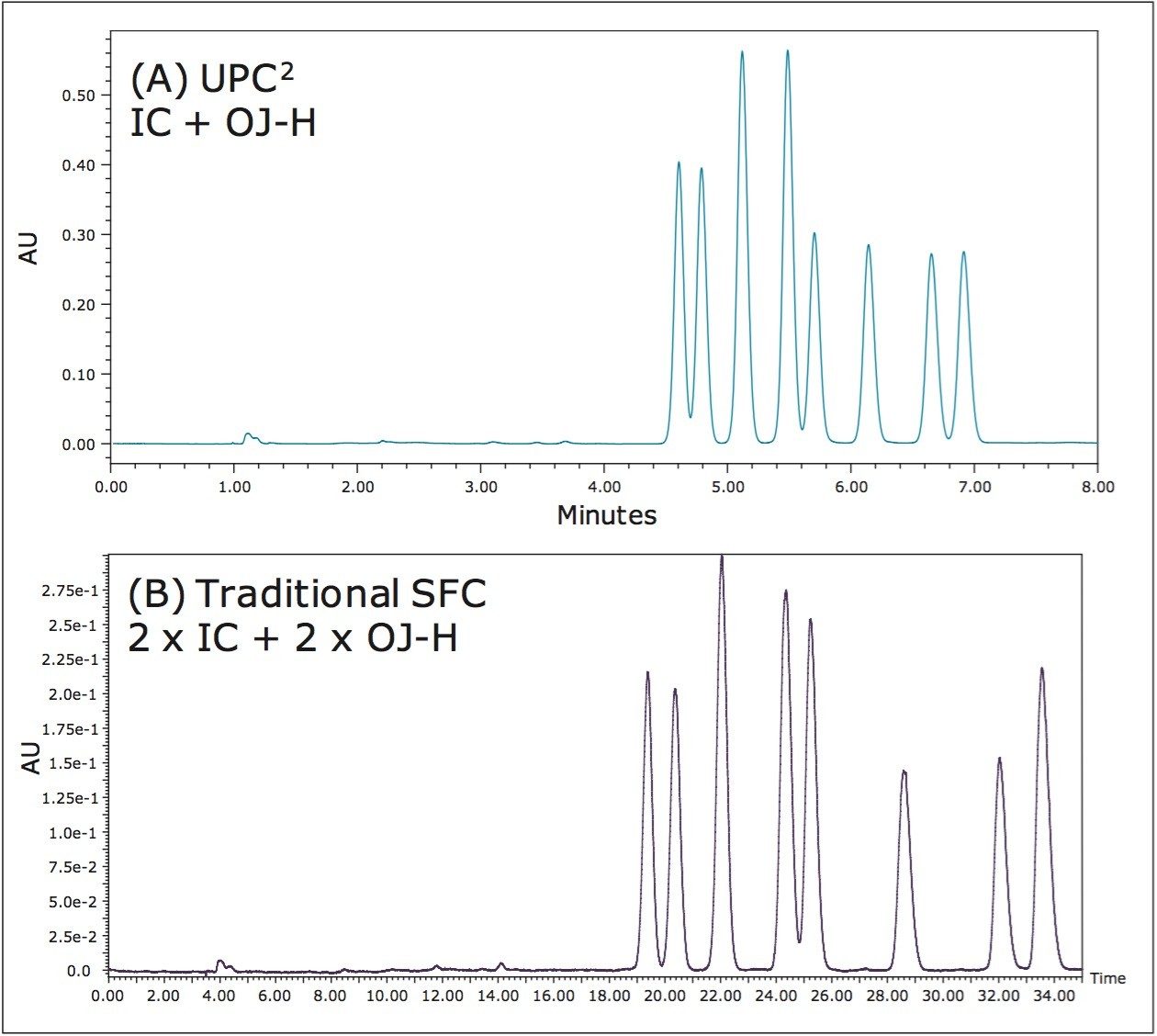
Figure 6 shows the diastereomeric separation of cyfluthrin, a pyrethroid with three chiral centers, on both UPC2 and traditional SFC systems. Similar separations were achieved on both systems. Only two coupled columns (300 mm) were used for the UPC2 system; whereas, four coupled columns with a total length of 800 mm were required on the traditional SFC system. The low extra-column volume of the UPC2 system greatly mitigates peak dispersion, resulting in sharper peaks, higher resolution, and significantly reduced analysis time (8 min using a UPC2 system compared to 35 min using a traditional SFC system). The separation of cyfluthrin also exemplifies another type of column coupling. Each column offered complementary separations. Cumulatively, the coupled columns of different chemistries resulted in the simultaneous separation of all eight isomers. The coupled columns in this case essentially altered the selectivity rather than increasing column length, as illustrated in Figure 5.
In this application note, we demonstrated some challenging chiral separations of pyrethroids with varying stereochemical complexity using a Waters ACQUITY UPC2 System. Due to the nature of chiral separations, it is important to systemically explore some secondary chromatographic parameters, such as temperature and flow rate, to achieve the desired resolution. The separations presented in this paper are thermally sensitive. Temperature optimization is a critical step for method development. The low viscosity of supercritical CO2 allows for facile column coupling, between identical or different column chemistries, for highly efficient chiral separations required for resolving pyrethroid isomers. Finally, low system volume and extra-column volume of the ACQUITY UPC2 System enables superior, faster, and more efficient diastereomeric separations of pyrethroids compared to traditional SFC.
720004530, December 2012