
This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrate the results obtained from the impurity analysis of estradiol with the ACQUITY UPC2 System are equal to or better than those achieved using the current USP method.
The UPC2 method used for the estimation of the chromatographic purity of estradiol was three times faster than the current normal phase HPLC method and reduced cost per analysis by more than 100 times.
Currently, the United States Pharmacopeia (USP) method for the estimation of chromatographic purity of estradiol utilizes a 4.6 x 250 mm silica column and a mobile phase consisting of 2,2,4-trimethylpentane, n-butyl chloride, and methanol 45:4:1 at 2 mL/minute. Since many laboratories have a desire to limit the use of aliphatic hydrocarbons and chlorinated solvents, alternative chromatographic techniques, like supercritical fluid chromatography (SFC) must be evaluated.
The Waters ACQUITY UPC2 System was used to develop a method for the evaluation of the chromatographic purity of estradiol. Results obtained from the UltraPerformance Convergence Chromatography (UPC2) method were directly compared to results obtained for the current USP method to detect estradiol impurities. The results from both techniques were similar with the UPC2 method showing adequate sensitivity to detect impurities in estradiol equal to those obtained from the normal phase HPLC USP method. In addition, when using UPC2, sample run time is reduced and overall cost per analysis (based on solvent usage and waste disposal costs) is significantly reduced.
|
The UPC2 method conditions were as follows: |
|
|---|---|
|
Column: |
ACQUITY UPC2 BEH, 2.1 x 150 mm, 1.7 μm |
|
Mobile phase: |
A=CO2 B=1:1 Methanol/2-Propanol |
|
Back pressure: |
130 bar/1880 psi |
|
Temp.: |
45 °C |
|
Detection: |
UV/PDA at 280 nm |
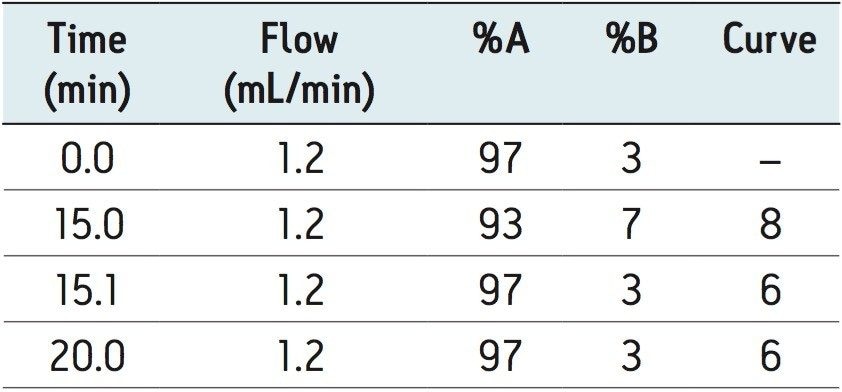
A sample of estradiol was prepared and analyzed using the current USP method as shown in Figure 1. The results of this analysis were used to compare with the results obtained in the method developed on an ACQUITY UPC2 System as seen in Figure 2 using the identical sample preparation.
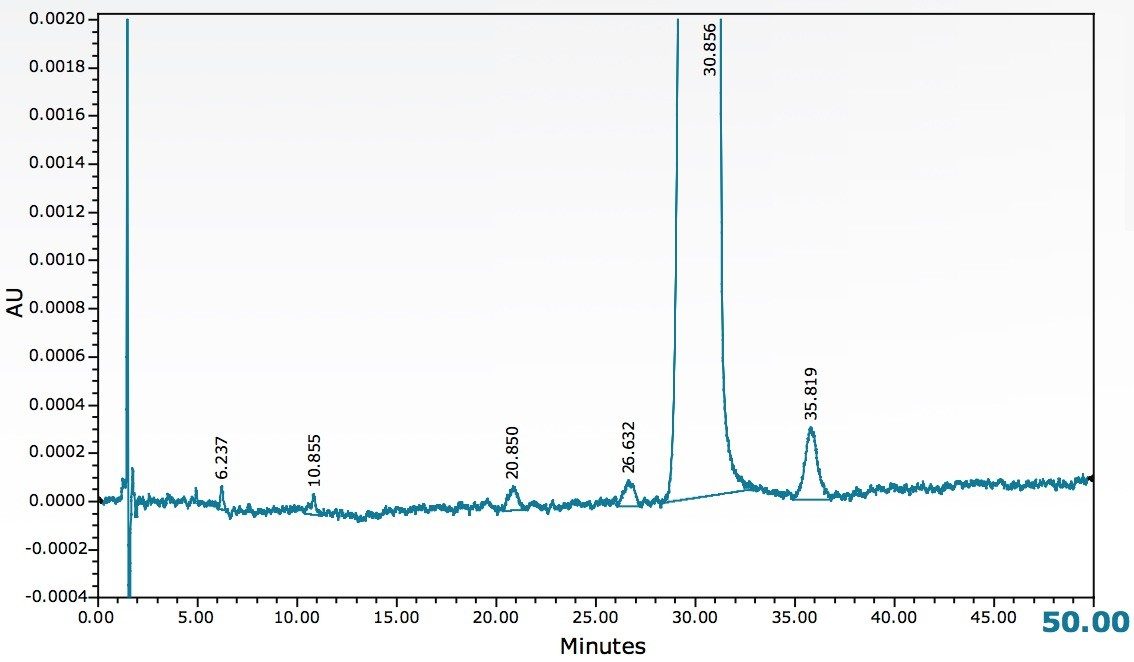
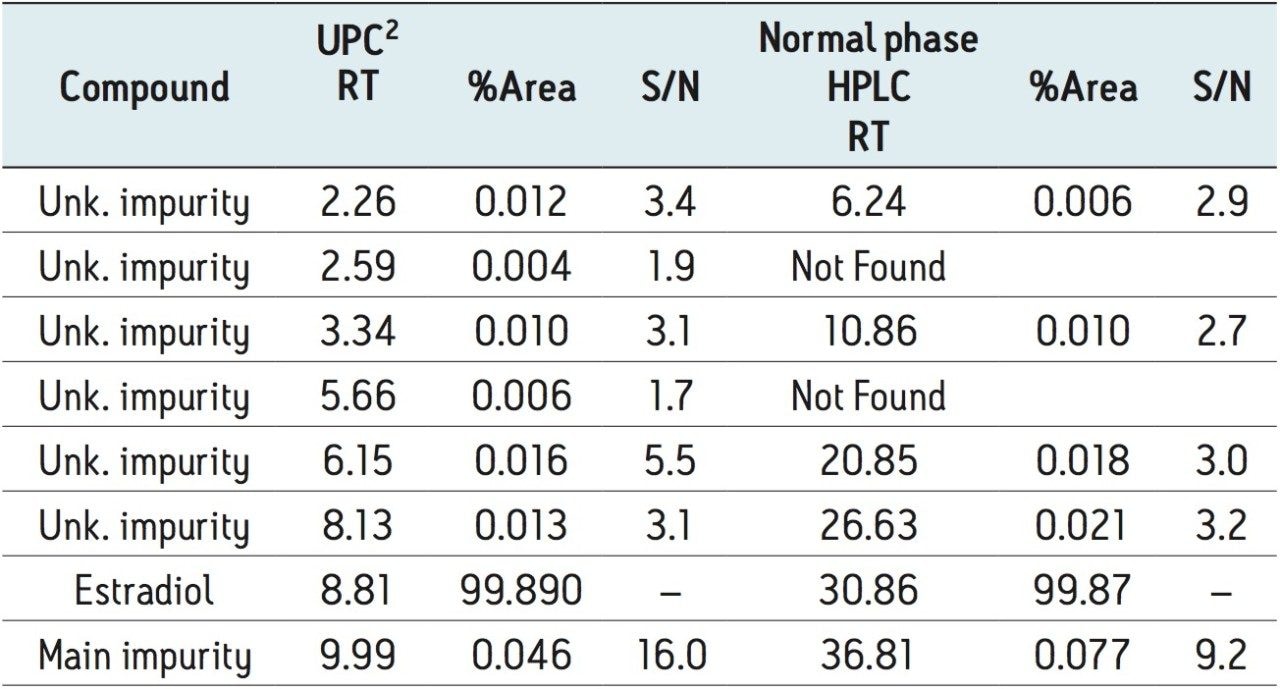
A comparison of results from the two methods is shown in Table 1. Both the normal phase HPLC and UPC2 methods detected at least five impurities below 0.1% (based on area). Signal to noise values for peaks in the range of 0.01% were all approximately 3:1 for both methods with the UPC2 results giving slightly higher values. The largest impurity (approximately 0.05% based on area) gave a signal to noise value of 16:1 for UPC2 and 9:1 for normal phase HPLC. These results clearly show that the ACQUITY UPC2 System can be used to successfully analyze minor impurities from estradiol. The run time of the UPC2 method was considerably shorter than the normal phase HPLC method (20 minutes compared to 60 minutes) resulting in an increase in lab productivity. An analysis of cost per run showed that the cost of solvent for the normal phase HPLC
A method for the estimation of the chromatographic purity of estradiol was developed, using the ACQUITY UPC2 System. This UPC2 method was three times faster than the current normal phase method from the USP. In addition to speed, this method reduced the cost per analysis by more than 100 times, primarily by reducing the need for aliphatic hydrocarbons and chlorinated solvents. Required sensitivity levels were achieved in the UPC2 method with impurities as low as 0.01% of the main peaks being easily detected. The ACQUITY UPC2 System is an ideal choice for laboratories looking for an alternative to conventional normal phase HPLC.
720004243, February 2012