The analysis of environmental samples is critical following suspected battlefield or terrorist use and in support of CWC monitoring for peacekeeping operations. A fast and easy analytical method for the detection of nerve agents, VX, GB, R-VX, GF, and GD is presented here. This method is suitable for the analysis of biological and environmental samples. Separation of the five compounds was achieved in less than 1.8 minutes, saving precious time when exposure is suspected in order to initiate counter measures more rapidly.
Saving precious time when exposure is suspected in order to initiate countermeasures more rapidly
Increased public concern regarding the use of chemical warfare has highlighted the need for a rapid, sensitive method for the detection and identification of the presence of chemical warfare agents. Although more than 160 countries have signed the Chemical Weapons Convention (CWC), which prohibits the development, production, stockpiling, and use of chemical weapons by the military1, there is a serious threat of their use in a terrorist attack or by countries that did not participate in the CWC.
Nerve agents are organophosphate-type compounds. The first class of nerve agents, the G-Series, were synthesized in Germany during World War II. The G-series consists of GA (Tabun), GB (Sarin), GD (Soman), and GF (Cyclosarin)2. Sarin gained notoriety in 1995 because of its use in the Tokyo underground terrorist attack3. The second more toxic series of nerve agents, the V-series, consists of VX, developed in the UK in the 1950s, and R-VX (Russian VX).
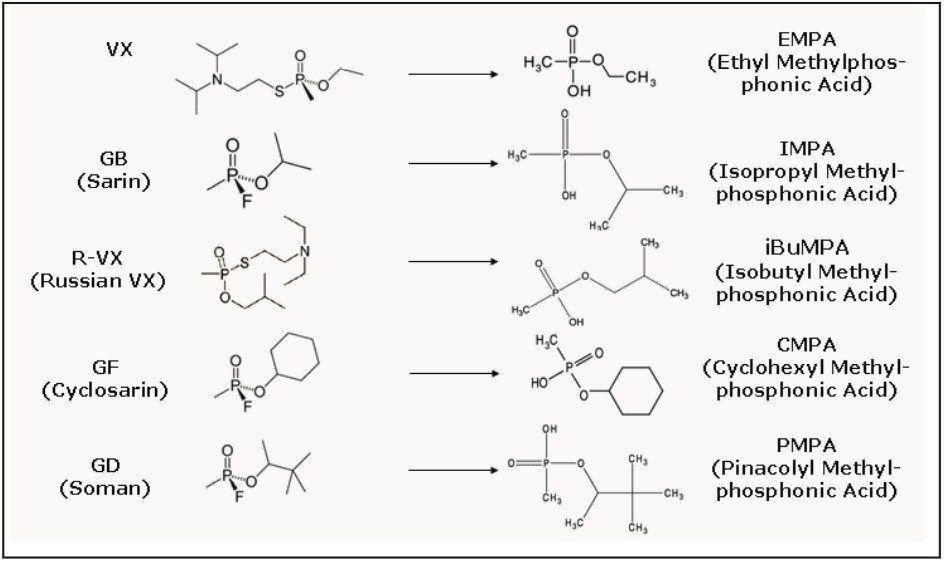
All nerve agents are extremely toxic, even in small amounts. One milligram of VX on the skin can be fatal. The mode of action of nerve agents in the body is to inhibit cholinesterase enzymes, causing the accumulation of acetylcholine: resulting in prolonged stimulation and paralysis of muscles4. In the body and in the environment, nerve agents metabolize/degrade rapidly to form the corresponding Alkyl Methyl/Phosphonic Acid (AMPA). Figure 1 shows the nerve agents and their degradation products.
A common antidote, atropine, is effective for both the G- and V-series nerve agents. Following exposure and the onset of symptoms, the antidote needs to be administered rapidly2. However, since the breakdown products are unique for each of the nerve agents, the analysis of plasma or urine samples of exposed individuals is helpful in identifying both the exposure agent and the degree of exposure.
The analysis of environmental samples is relevant following suspected battlefield or terrorist use and in support of CWC monitoring for peacekeeping operations. The need for test methodology for saltwater samples can also become necessary as ocean dumping of steel containers containing nerve agents was common in various parts of the world until the 1970s. The leakage of nerve agents into the ocean and the effect on the ecosystem is currently unknown2.
A fast, easy analytical method for the AMPAs of the nerve agents, VX, GB, R-VX, GF, and GD, is presented here. This method is suitable for the analysis of biological and environmental samples, following appropriate sample preparation, without the need for derivatization. The analysis of these compounds is typically performed by HPLC-MS/MS or GC-MS with analysis times of more than 20 minutes5,6. Five nerveagent degradation products were analyzed using UPLC-MS/MS with the Waters ACQUITY UPLC TQD System (Figure 2) in less than 1.8 minutes; the total cycle time was four minutes.

The AMPAs are low molecular weight, polar compounds that are difficult to retain on conventional reverse phase (RP) HPLC columns. Also, the retention of highly polar analytes by RP chromatography often requires a non-volatile, highly aqueous mobile phase, which is not ideal for electrospray MS. HILIC is a variation of normalphase chromatography with polar stationary-phase columns and high organic-content mobile phases. The main advantage overconventional normal-phase chromatography is the use of solvents which are miscible in water. HILIC is ideal for the direct analysis of plasma samples prepared by protein precipitation or environmental samples prepared by SPE. Both produce highly organic eluents which are compatible with the highly organic-gradient starting conditions: which eliminates the need for time-consuming evaporation and reconstitution steps7.
Standards of the following alkyl methyl phosphonic acids were obtained from Cerilliant Corporation: pinacolyl methyl phosphonicacid, isobutyl methyl phosphonic acid, cyclohexyl methyl phosphonic acid, and isppropyl methyl phosphonic acid. Ethyl methyl phosphonic acid was purchased from Sigma Aldrich.
|
LC system: |
ACQUITY UPLC System |
|
Column: |
ACQUITY UPLC BEH HILIC 2.1 x 100 mm, 1.7 μm |
|
Eluent A: |
10 mM Ammonium acetate in 90/10 acetonitrile/water |
|
Eluent B: |
10 mM Ammonium acetate in 10/90 acetonitrile/water |
|
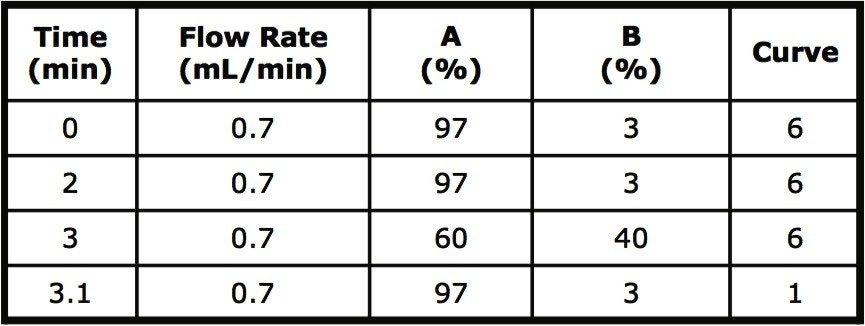
Gradient: |
See table 1 |
|
Run time: |
4 minutes |
|
Column temp.: |
35 °C |
|
Injection volume: |
10 μL |
|
MS system: |
ACQUITY TQD |
|
Ionization mode: |
ES- |
|
Capillary voltage: |
3.6 kV |
|
Source temp.: |
150 °C |
|
Desolvation temp.: |
400 °C |
|
Desolvation gas: |
800 L/hr |
|
Cone gas: |
50 L/hr |
|
Collision gas: |
Argon at 3.2 x 10-3 mBar |
|
Acquisition mode: |
MRM |
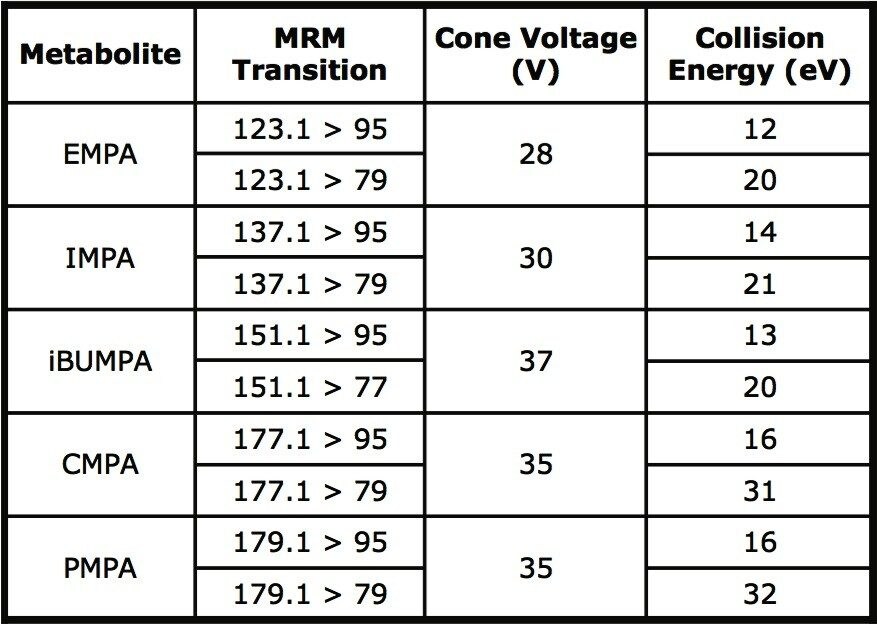
Two Multiple Reaction Monitoring (MRM) transitions were obtained for each of the five metabolites. The optimum cone voltage was found for each compound and the collision energy was optimized for each of the product ions. The MRM transitions are listed in Table 2 along with their respective optimized cone voltages and collision energies.
Data acquisition was performed using Waters MassLynx v.4.1 Software.
The Waters UltraPerformance LC technology enables faster and higher peak capacity separations than conventional HPLC. Using UPLC, baseline separation of five AMPAs was achieved in less than 1.8 minutes, compared with the up to 20 minute literature reports of HPLC separations5,6. Figure 3 shows the UPLC-MS/MS chromatogram of a 10 pg/μL standard.
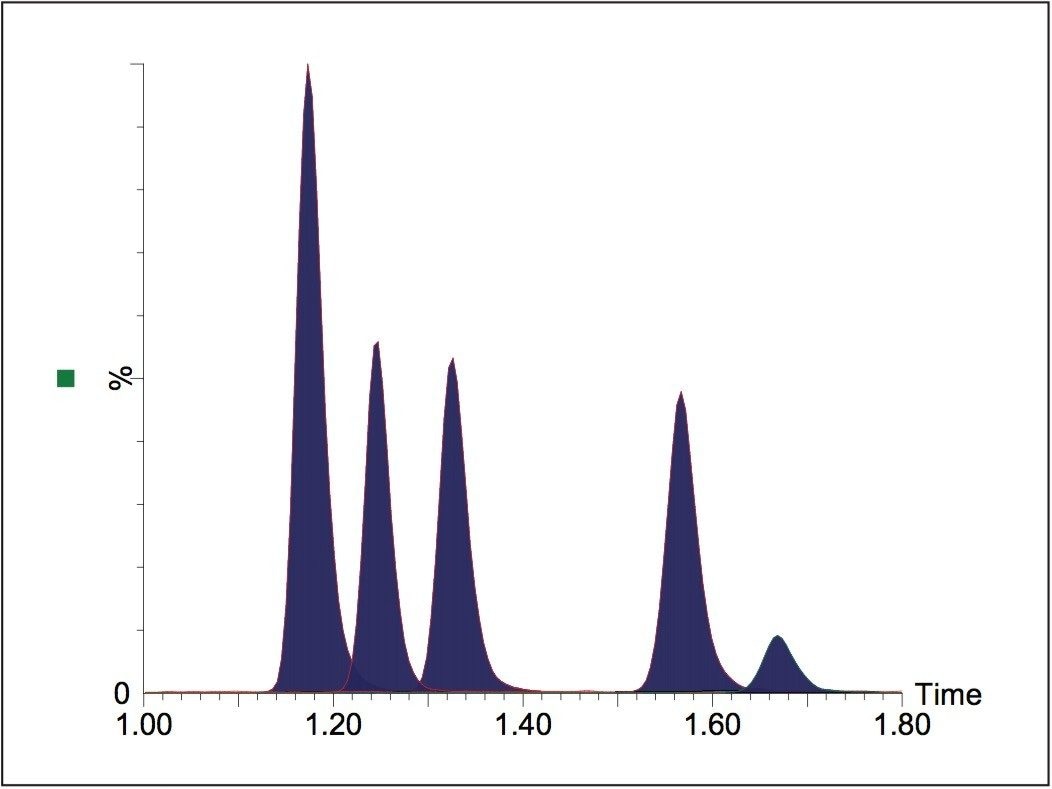
Two Multiple Reaction Monitoring (MRM) transitions were monitored for each of the AMPAs. The primary transitions were used for quantification and the secondary transitions for confirmation purposes. Waters TargetLynx Application Manager Software was used to calculate the ion ratio between the primary and secondary transitions and for calibration and quantification.
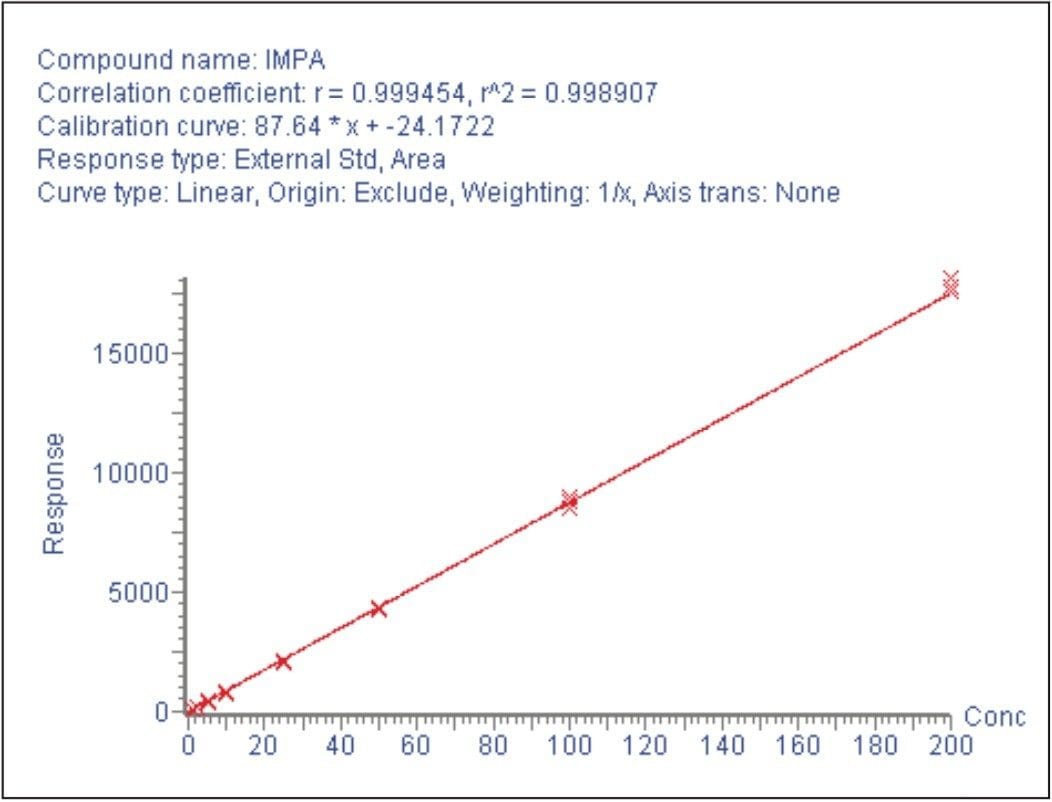
Calibration curves for all five compounds were linear over the range 0.5 to 200 pg/μL with acceptable regression coefficients (see Table 3). Figure 4 shows a representative calibration curve for the compound IMPA.
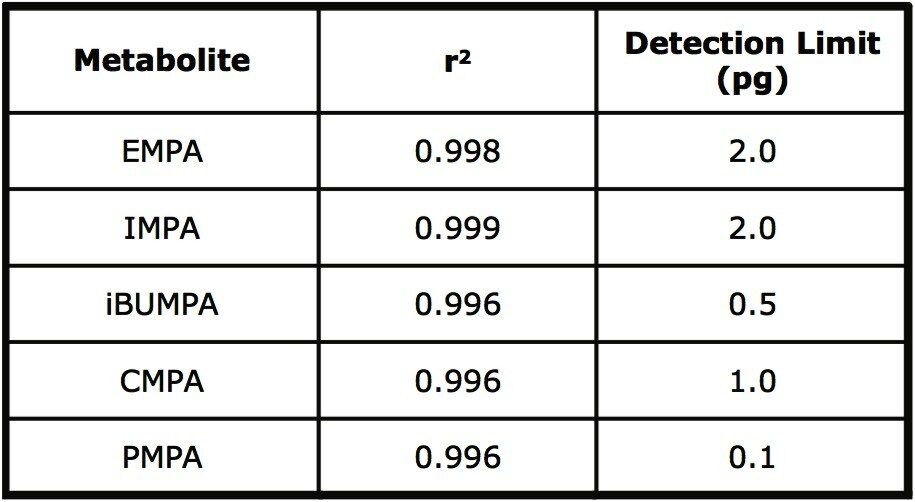
The detection limits for the five AMPAs were investigated and the results are shown in Table 3. The detection limit was defined as S/N value of 3:1 and reported as pg injected on column. Reported relevant concentrations range from 4–1000 μg/g in solid media5,8 and 1–200 μg/L in clinical samples9.
Quantification and confirmation of the presence of the nerve agents VX, GB, R-VX, GF, and GD were achieved in one analysis. This was accomplished by applying a fast, sensitive UPLC-MS/MS method for the analysis of five nerve agent degradation products. Separation of the five compounds was achieved in less than 1.8 minutes. The method is based on Hydrophilic Interaction Chromatography, which permits the elimination of time-consuming evaporation and reconstitution steps following SPE or protein precipitation: saving precious time when exposure is suspected in order to initiate counter measures more rapidly.
720002334, November 2007