
The unique EBE design concept of the Waters Micromass AutoSpec Ultima NT mass spectrometer offers not only exceptional sensitivity for trace compound analysis, but also powerful facilities for the analysis of complex mixtures.
The unique EBE design concept of the Waters Micromass AutoSpec Ultima NT mass spectrometer offers not only exceptional sensitivity for trace compound analysis, but also powerful facilities for the analysis of complex mixtures. Linked scanning, in which 2 or more of the instrument's analyzer segments are simultaneously scanned, facilitates the elucidation of the relationships between precursor ions and productions formed as a consequence of decomposition in the first field free region (FFR1) of the instrument. This enhanced selectivity provides a significant reduction in spectral complexity and provides structural information, giving rise to more straightforward spectral interpretation.
Ions that are created and accelerated away from the ion source can then fragment either by natural decay or be induced to fragment by use of a collision gas. These resulting fragmentation patterns give rise to the Metastable Ion (MI) spectrum, which can then be studied by the linked scanning technique.
The MI spectrum of these fragmentations can be obtained by scanning the instrument in a 'defocussed' mode, which separates the MI peaks from the normal ion peaks in a fully focussed spectrum. Several scanning modes, in which two of the three fields and voltages (B, E, and V) are varied simultaneously, have been devised to produce the required MI spectrum, but this document discusses only three modes in which V is held constant. These are :
a) The product ion scan (B/E) which records all fragmentation products, m2+, of a given precursor, m1+.
b) The precursor ion scan (B2/E) which records all precursors, m1+, of a given fragmentation product ion, m2+.
c) The constant neutral loss scan (CNL) which records all precursor ions, m1+, which give rise to products formed by the loss of a given neutral mass, mn.
When an ion, of mass m and charge z, is accelerated away from an ion source through a potential V it gains an energy ( E) described by the following equation:
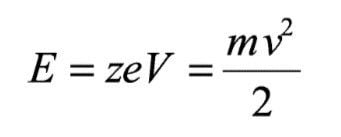
Where e is the charge of an electron and v is the velocity of the ion. When the ion is exposed to a magnetic sector of field (B) and radius (R), it is deflected according to the Lorentz equation:
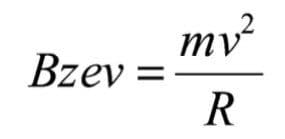
If the magnetic field is set to an appropriate value, the ion of mass m is passed through the magnetic sector radius R and will subsequently be detected. This is the principle of operation for a magnetic sector mass spectrometer.
By substituting for velocity (v) in equation (2) with a value derived from equation (1), the following expression can be obtained:
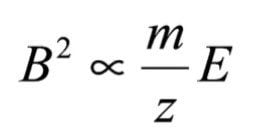
A fragmentation of a singly charged ion (z =1) can be represented by the following equation:
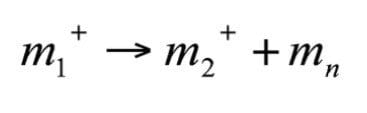
Where mn represents the mass of the neutral fragment. Linked Scanning is used to study reactions occurring in the region prior to the electric and magnetic sectors but after the ion has been fully accelerated away from the source. This region is known as the first Field Free Region (FFR1).
In the process of being accelerated from the source, the precursor ion (m1) gains an energy of E1, as defined by equation 1. If this ion then fragments after leaving the source, its energy will be transferred to the product ion (m2) and neutral fragment in proportion to their masses.
For the product ion, m2, to be transmitted through the electric sector, it must be set to a field strength of:
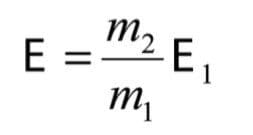
Where E1 is the electric sector energy required to pass ions of energy E1, i.e. those which have been accelerated through the source potential V, and not subsequently fragmented.
For this product ion to then be passed through the magnetic sector, the field strength must be set to a value given by:
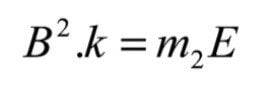
Where k is a constant of proportionality from equation (3). By using the expression for E given in equation (5), this field can be expressed as:
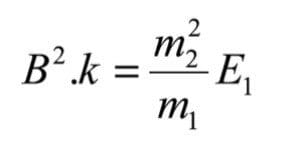
This same B field would pass a hypothetical ion, of mass m*, formed in the source, and not subsequently fragmented:
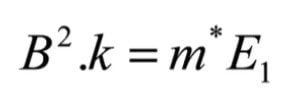
By combining equations (7) and (8)
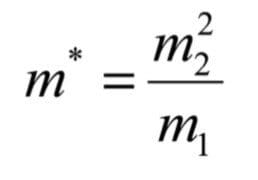
This value of m* is often referred to as the 'metastable mass'. It is a convenient means of describing the strength of the magnetic field as it represents the mass that would normally be passed by the magnet at full accelerating voltage. This equation provides the basis for deriving the relationships required to perform the linked scans for the analysis of FFR1 fragmentations.
Magnetic sector mass spectrometers discriminate mass by merit of the degree of deflection experienced by an ion when subjected to a magnetic field. The magnetic field is a momentum analyzer, that is it will discriminate on the basis of a charged particle's mass and velocity. The ion's velocity is given by subjecting it to an accelerating potential. Singly charged ions of all masses experience the same force and hence gain the same kinetic energy. They will therefore have velocities dependent on their mass. The exact force experienced by the ions will vary subtly, due to a number of effects such as spatial distribution, resulting in the ions having an energy spread.
By the inclusion of an electric sector in the ions' flight path it is possible to focus ions of varying energy and improve the attainable resolution of a magnetic sector mass spectrometer. A mass spectrometer with both magnetic and electric sector fields is said to be “double focusing.” Earlier double focusing mass spectrometers had the electric sector preceding the magnetic sector. This allows the ions to be energy focused prior to being subjected to the magnetic field. This type of field arrangement is known as conventional or forward geometry. Another configuration is with the electric sector after the magnetic sector. This is known as reverse geometry.
The AutoSpec Ultima NT is unique in that it has an E-B-E geometry. The electric sector is split and placed either side of the magnet. This has the benefit of improving the transmission of ions, at low and high resolution, giving the AutoSpec Ultima NT its unrivalled sensitivity.
It is possible to perform linked scans on any geometry of double focusing magnetic sector mass spectrometer. MIKES can only be performed on a reverse or EBE geometry machine as the precursor ion needs to be selected prior to energy analysis. On the AutoSpec Ultima NT, MIKES is performed in the third Field Free Region (FFR3).
With forward and reverse geometry machines, it is possible that artefact peaks will be detected. These are interference peaks resulting from a two or greater stage fragmentation reaction. For a reverse geometry machine, it is always possible for the magnetic field to pass product ions which have the correct momentum but which have arisen from the fragmentation of an ion other than the precursor ion of interest. These ions should be automatically rejected though because they will not have the correct energy to pass through the electric field. However, if the ion fragments a second time, between the B and E fields, it is possible that the resultant ion will have the correct energy to pass through the electric sector to the detector. This may mislead one to think the detected peak is product of the precursor ion being studied.
The same problem is an issue for forward geometry machines. In this case, the first fragmentation yields an ion with the correct energy to pass through the electric sector. The second fragmentation results in the product ion having the required momentum to pass through the magnetic sector to the detector. With a reverse geometry machine this type of two stage fragmentation has to begin with a precursor ion of a higher mass than the ion of interest. For this reason, artefact peaks do not tend to be a big problem. For a forward geometry machine, however, the precursor ion would have to be of a lower mass than the ion of interest and as such can be a serious issue, especially when using a GC.
The AutoSpec Ultima NT's EBE geometry prevents these two stage fragmentations from being a problem. Because there is an energy filter before and after the magnetic sector, only ions with the correct energy and momentum will pass through all three fields to the detector. Secondary fragmentations between the electric and magnetic sectors will cause the ion to be rejected. For this reason the AutoSpec Ultima NT provides artefact-free linked scanning.
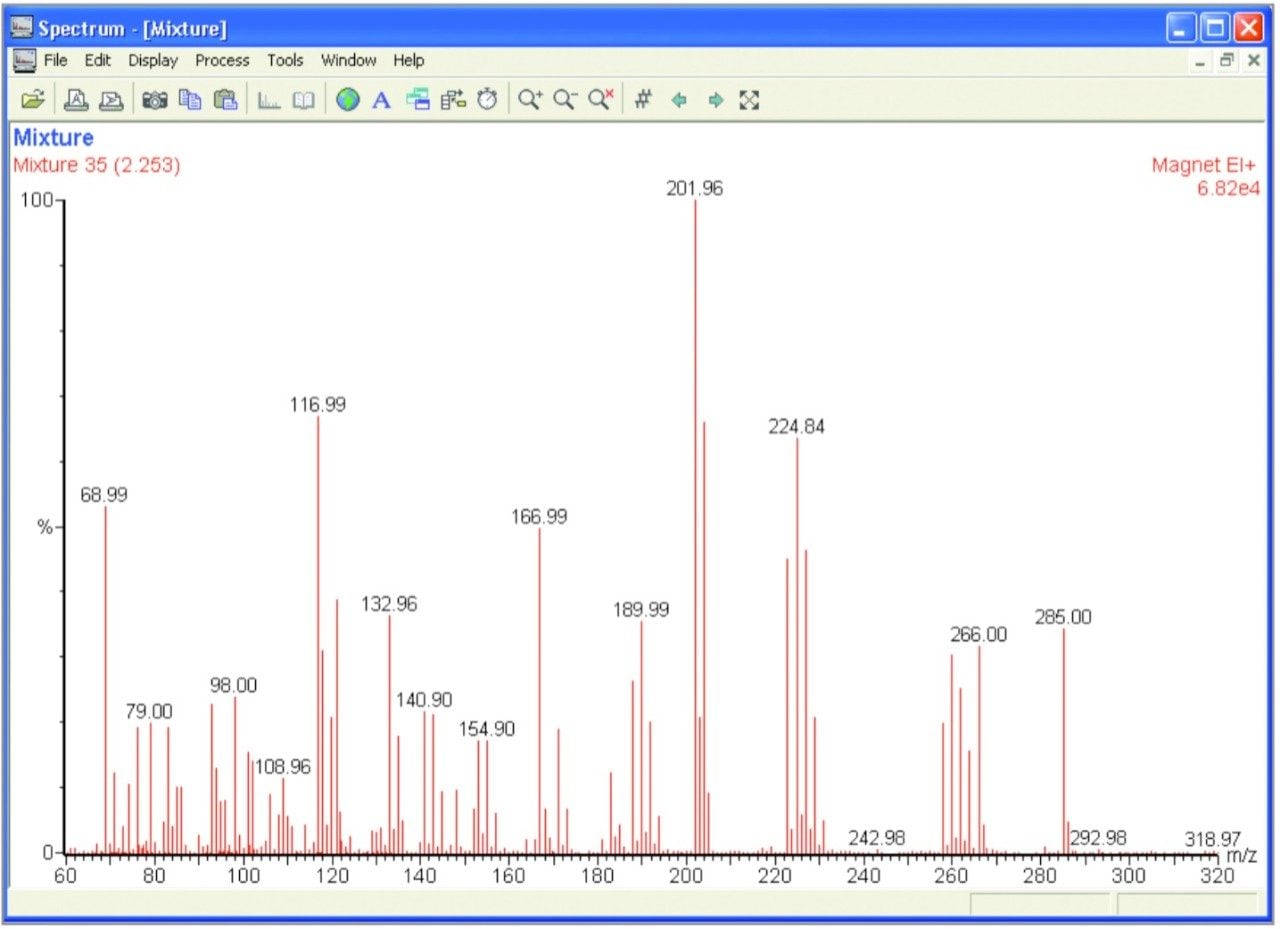
In order to demonstrate the capabilities of linked scanning, a specific compound was analyzed within a complex mixture. Tris( Trifluoromethyl)- Triazene was added to a mix consisting of heptacosa, perfluorokerosene, hexachlorobutadiene and chloropentaflorobenzene. A full magnet scan of the mixture can be seen in Figure 1.
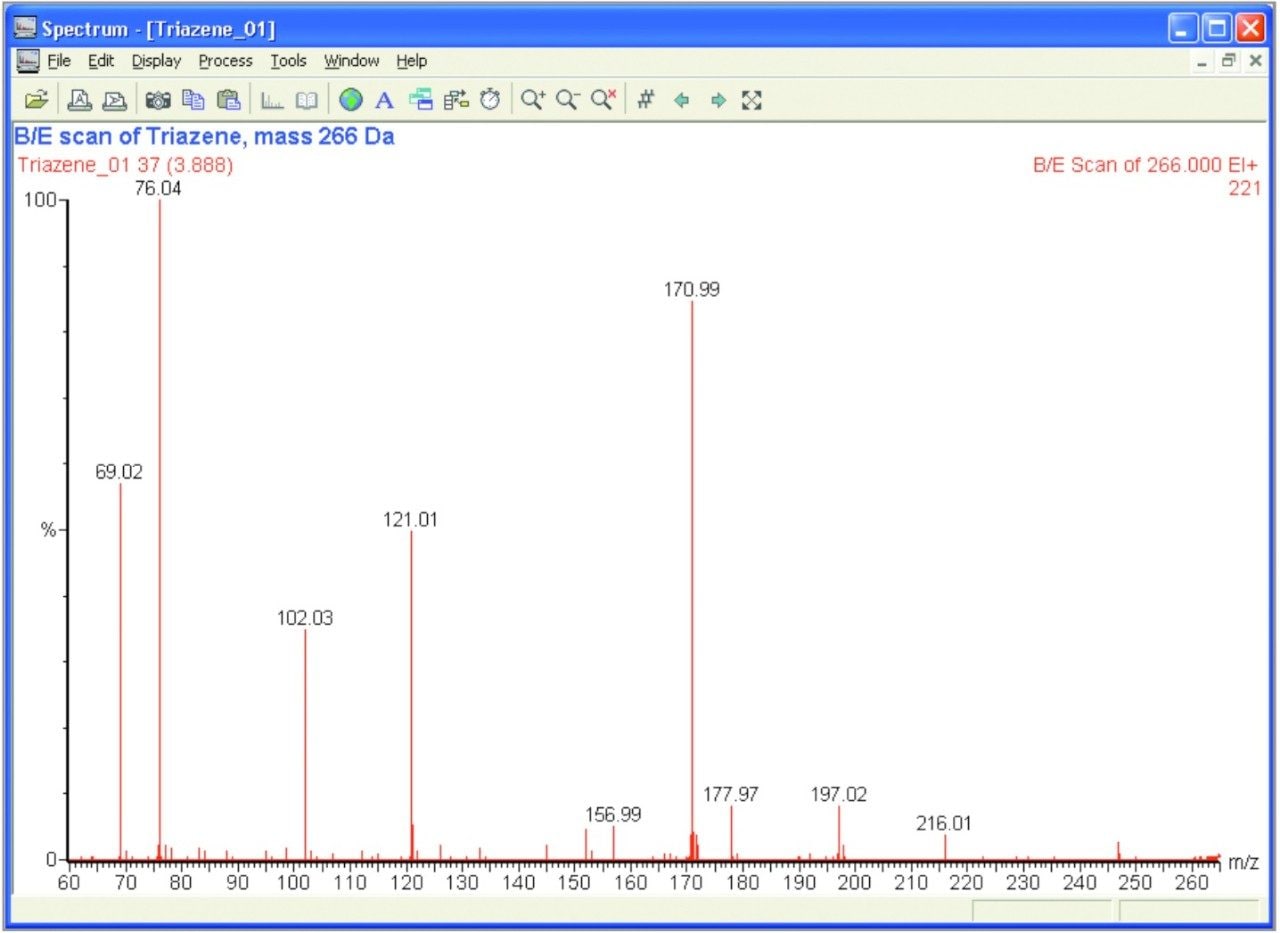
A B/E scan of mass 266 Da was conducted in o rder to study the fragments of one of the main Triazene peaks. The results of this can be seen in Figure 2.
A MIKES scan of Benzene was conducted in order to demonstrate how this technique can be used to derive structural information through the study of the molecule's Kinetic Energy Release Distribution (KERD). Figure 3 shows the peak which results from the fragmentation of a doubly charged benzene ring. The loss of a carbon atom results in an energy release, giving the fragments an additional kinetic energy component. This gives the observed peak its width.
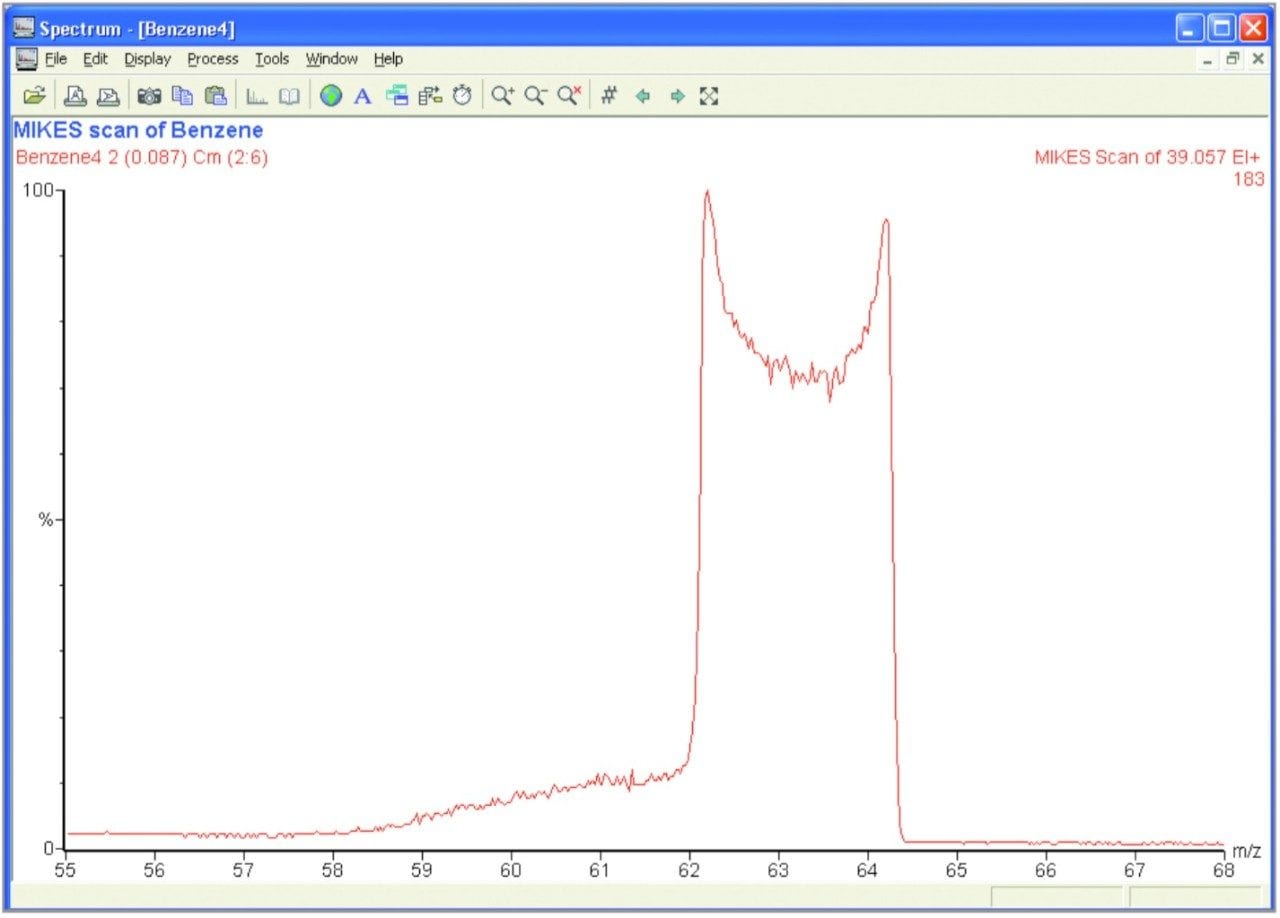
The discoid shape in the centre of the peak is the result of a combination of the energy release being in a sphere and the fragment ions passing through a vertically parallel electric sector. When a fragment ion gains the kinetic energy component exactly perpendicular to the ion's direction of propagation, it will appear at the exact centre mass as it will not have gained any energy in the plane being analyzed. However, the ion will be more likely to collide with the electric sector plates and be neutralised. This results in the lower abundance of ions with the exact centre mass (energy) of the peak and hence the observed shape. This only occurs for relatively high bond energy releases, such as with Benzene.
Using the following equation it is possible to calculate the energy release from the observed peak width.
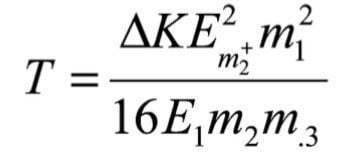
Where T is the bond energy, E1 is the precursor ion energy (equal to the accelerating voltage multiplied by the ion's charge) and KE is the kinetic energy. MIKES produces a linear energy spectrum and therefore the energies can be derived from the apparent mass of the peak using the following relationship:
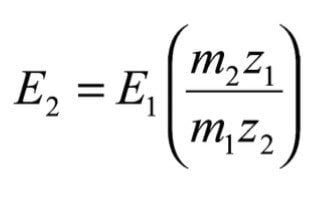
From the width of the peak observed in Figure 3 and using equation (15) we get a value of kinetic energy spread (DKE) of 247 eV. Using equation (14) this yields a bond energy of 2.7eV.
(Product ion scan)
The magnetic sector field strength, B, and the electric sector field strength, E, are scanned simultaneously, while holding the accelerating voltage, V, constant, so as to maintain the ratio B/E constant. This constant value is determined by the ratio of the two field strengths required to transmit main-beam ions of pre determined mass/ charge ratio. These preselected main-beam ions are the precursor ions (m1) whose fragment ion spectrum is required.
Using equations (5) and (9), E can be expressed in terms of m* and E1 for a given constant m1:
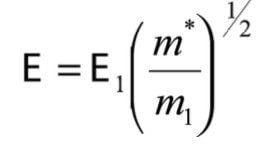
and substituting for m* from equation (8), the required value of E2 is:
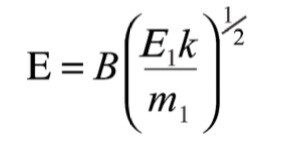
As E1, m1 and k are constants for this scan it can be seen that E must be maintained proportional to B such that B/E remains constant.
(Precursor ion scan)
The accelerating voltage is fixed and the magnetic field, B, and the electric field, E, are scanned simultaneously so as to maintain B2/E constant. This constant value corresponds to the ratio of the two fields which transmits main-beam ions of pre determined mass/charge ratio; these preselected main-beam ions are the fragment ions (m2) whose precursor ion spectrum is required. Using equation (5) and (9), E can be expressed in terms of m* and E1 for a given constant m2
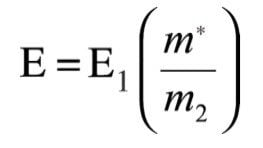
and substituting for m* from equation (8), the required value of E2 is:
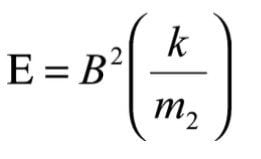
As m2 and k are constants for this scan it can be seen that E must be maintained proportional to B2 such that B2/E remains constant.
(Constant neutral loss scan)
The magnetic sector field strength, B, and the electric sector field strength, E, are scanned simultaneously, while holding the accelerating voltage, V, constant, so as to maintain the ratio B [1-(E /E1)]1/2/E constant. This constant value is equal to B3/E1, where E1 and B3 a re respectively the electric and magnetic sector fields required to transmit m3+ ions in the main ion beam; m3 represents the mass (m1- m2) of the selected neutral fragment m3 whose precursor spectrum is required.
MRM is a linked scan-derived function whereby the magnetic and electric fields are jumped through a series of values corresponding to specific reactions. The values of the B and E fields are derived from the supplied precursor and product masses and the linked scan equations above (specifically equations 5 and 9).
Mass-analyzed Ion Kinetic Energy Spectrometry (MIKES) is a technique which can be used to derive structural information about a given molecule. The technique involves selecting a precursor ion mass with the magnetic sector and fragmenting it in the region after the magnetic field and prior to the electric field. The resultant fragment ions' kinetic energy distribution is studied by scanning the second electric sector.
When the precursor ion fragments, either naturally or induced via a collision gas, the energy of the bond which has been broken will be transferred to the fragment particles. This energy release willresult in an additional velocity component being superimposed on the fragment ion's current trajectory. The direction of this additional velocity component will be dependent on the precursor ion's orientation at the point of fragmentation, and is therefore random.
If the kinetic energy release (KER) is in the same direction as the ion trajectory through the sector, the product will have a higher energy and appear earlier in the kinetic energy spectrum. Conversely, if the KER results in the additional velocity being against the direction of propagation of the fragment ion then it will have a lower energy and appear later in the energy spectrum. There will be a continuous distribution of energies detected between these two extremes. Thus the shape of the detected MIKES peak is actually a Kinetic Energy Release Distribution (KERD). The width of this peak can be used to infer the bond energy of the precursor ion.
A further analysis technique is available which is complementary to MIKES - Neutralization Re-ionization Mass Spectrometry or NRMS. The precursor ion is selected with the magnet and is then fragmented, usually with a collision gas. A high voltage deflection lens is then used to filter out all the charged species. The neutral species will pass through this lens under their own momentum. The neutral fragments are then re-ionised using second collision gas and the resultant ions are studied by scanning the second electric sector, as for MIKES. NRMS provides a method for creating and studying species which could not otherwise be formed in or analyzed by mass spectrometry, and allows even more information to be elucidated about the structure of a compound.
As can be seen by a comparison of Figures 1 and 2, linked scanning provides a means of extracting structural information of a specific compound even in complex mixtures. Further information can be obtained using the MIKES technique. The unique geometry of the AutoSpec Ultima NT provides flexibility and sensitivity making it the ideal choice for research and trace analysis.
720000844, May 2004