This application note demonstrates the capability of Waters bioanalysis solutions to provide the user with the ability to achieve high sensitivity with challenging and complicated samples, while addressing several other key challenges such as robustness, reproducibility, precision, and regulatory concerns.
Misoprostol is a synthetic analog of natural prostaglandin E1. It produces a dose-related inhibition of gastric acid and pepsin secretion, and enhances mucosal resistance to injury. It is an effective anti-ulcer agent and also has oxytocic properties having the chemical name methyl 7-[(1R, 2R, 3R)-3-hydroxy-2-[(1E)-4-hydroxy-4-methyloct-1-en-1-yl]-5-oxocyclopentyl]heptanoate and the chemical structure shown in Figure 1. Misoprostol is a light yellow viscous liquid with a molecular weight of 382.5341, and the empirical formula is C22H38O5. It is soluble in water, freely soluble in DMSO, alcohol, and dimethylformamide.

Misoprostol is rapidly and extensively hydrolyzed to its biologically active metabolite, misoprostol acid, in the gastrointestinal tract after oral administration. Unchanged misoprostol cannot be detected in the plasma even at 5 min after oral dose.1,2 Misoprostol has been approved in more than 85 countries for the prevention and treatment of gastric ulcers and is also frequently used in the field of obstetrics and gynecology.3 The therapeutic dose of misoprostol given orally is not more than 0.8 mg/day and the maximum plasma concentration of the active compound misoprostol acid in human plasma is less than 1.6 ng/mL.4-7 Owing to the low plasma concentration of misoprostol acid, a sensitive and simple analytical method is needed for its determination and quantification.
Several methods for the determination of misoprostol acid in biological matrices have been reported including radioimmunoassay, GC with electron capture mass spectrometry, or tandem mass spectrometry detection, and LC with mass or tandem mass spectrometry. However, the low plasma concentration of misoprostol acid demands for a more sensitive method compared to what has been observed with other methods. In this application note, we report quantification of misoprostol acid at an LLOQ concentration of 5 pg/mL. This highly sensitive assay for misoprostol acid is developed using the Waters Regulated Bioanalysis System Solution, comprising best-in-class chemistries for sample preparation and column chemistries, ACQUITY UPLC System, and Xevo TQ-S Tandem Quadrupole Mass Spectrometer.
The samples were isolated using solid phase extraction (SPE) employing a Oasis MAX 1 mL, 30 mg Cartridge. A 500 µL aliquot of plasma was diluted with an aqueous buffer solution and loaded onto an SPE cartridge previously conditioned with organic solvent and water. Plasma samples were washed with aqueous buffer solution followed by organic solvent. The samples were eluted with acidic organic solvent and transferred to an autosampler vial for LC-MS/MS analysis.
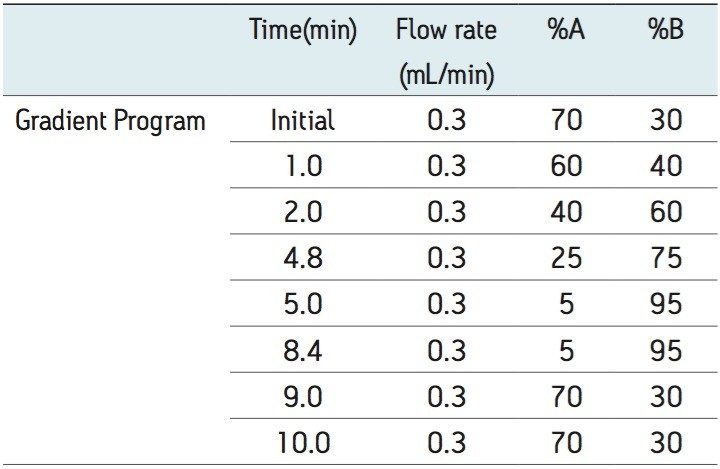
The extracted samples were analyzed by reversed phase gradient chromatography employing an acidic aqueous buffer and mixture of methanol and acetonitrile as the organic solvent. Chromatographic separation was performed on an ACQUITY UPLC System equipped with a binary solvent manager, column manager, and sample manager. Analyte was eluted at 70% organic over a 10 min run time under gradient conditions, shown in Appendix, Table 1. The separations were performed on an ACQUITY UPLC BEH C18 1.7 µm 2.1 x 150 mm Column and the temperature was maintained at 50 °C. Analyte was monitored by negative ion electrospray (ESI -) MS/MS using a Waters Xevo TQ-S Tandem Quadrupole Mass Spectrometer. Detection was performed using the transition 383.4 → 117.0.
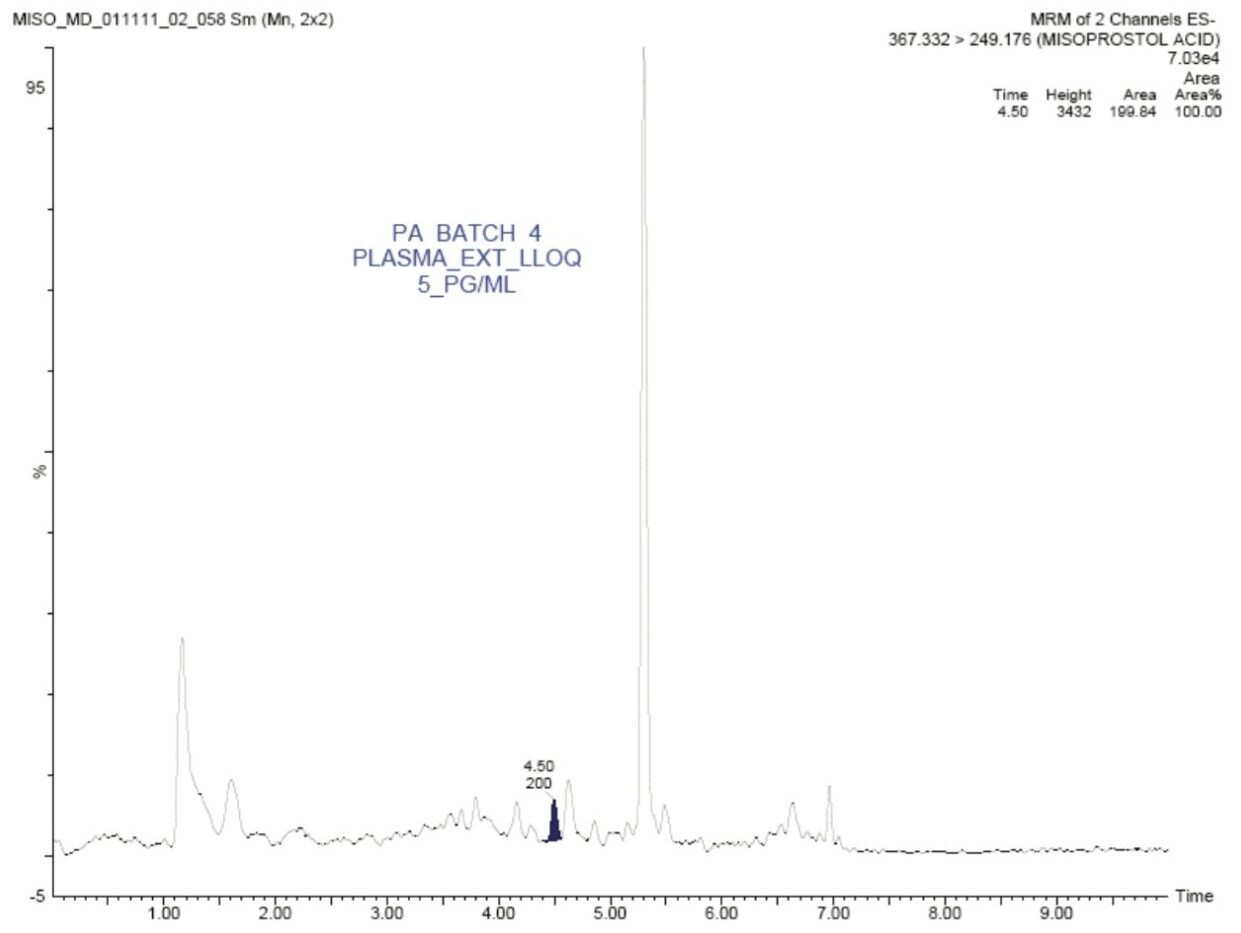
In this application note, misoprostol acid was quantified with an LLOQ of 5 pg/mL, and with a S/N ratio of 13:1, shown in Figure 2.
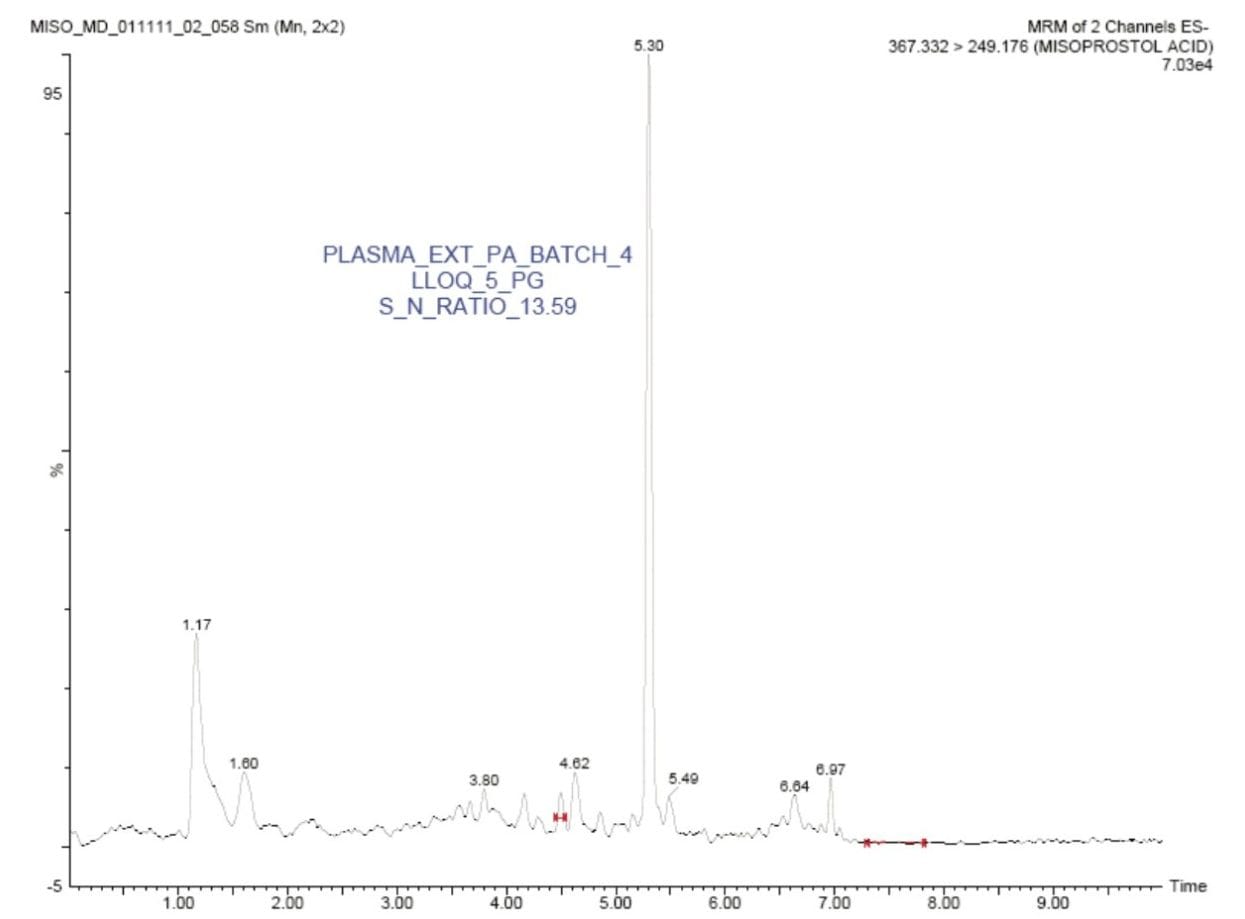
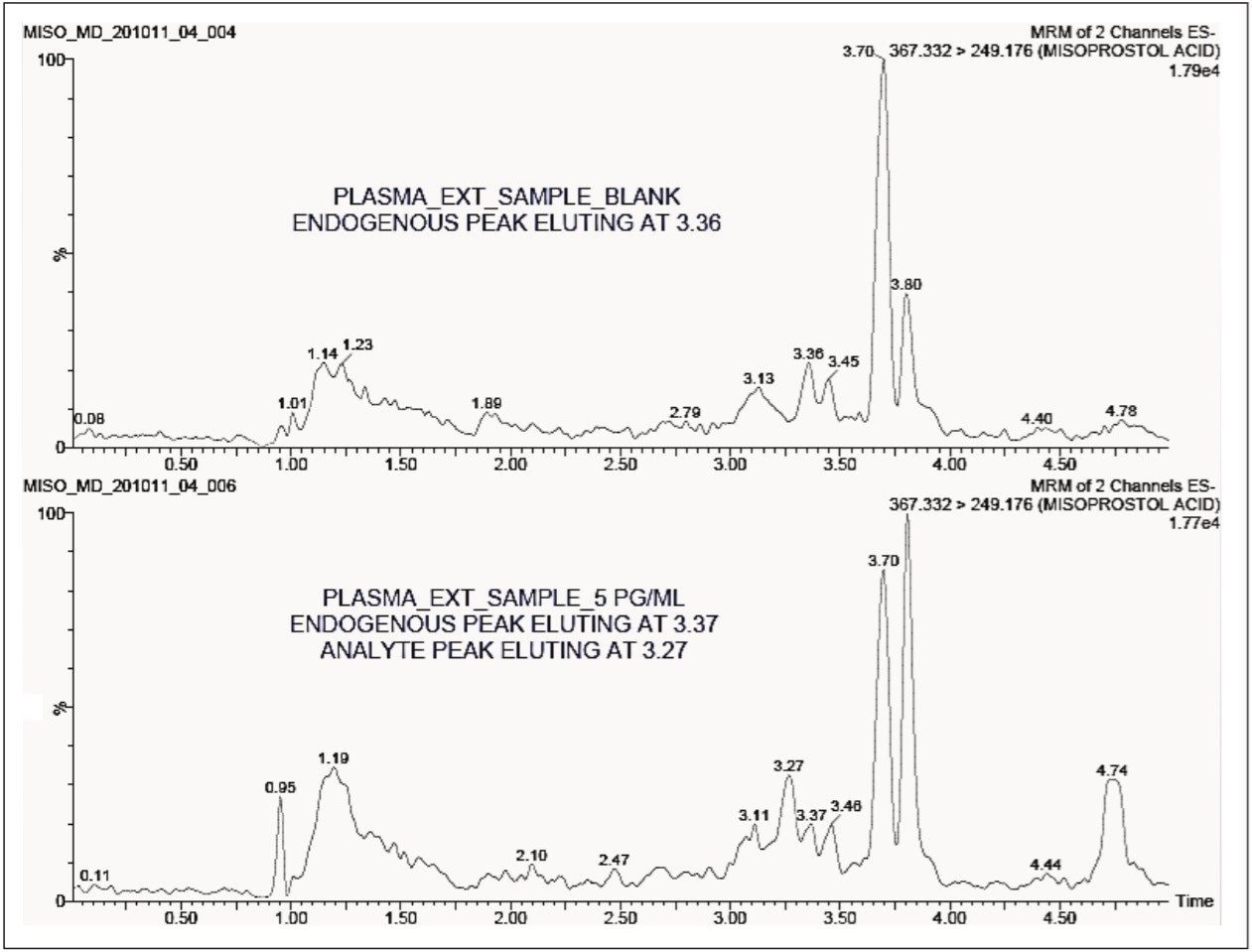
The standard chromatographic method resulted in poor resolution of signals from the analyte of interest and endogenous components, shown in Appendix, Table 1. As shown in Appendix, Figure 1, the analyte of interest elutes at 3.27 mins, while the endogenous components eluted at 3.36 mins. The modified chromatographic method used in this study gave excellent resolution for misoprostol acid from endogenous components in the samples, shown in Figures 3, 4, and 5. Target analyte and internal standard (IS, diclofenac) eluted with retention times of 4.50 and 5.45 min, respectively. Such a high-resolution separation was facilitated by the outstanding performance of the ACQUITY UPLC System equipped with an ACQUITY UPLC BEH C18 1.7 µm Column. The Xevo TQ-S Mass Spectrometers are equipped with a novel StepWave ion guide, which enables improved ion sampling in the source and ion transfer efficiency. This new ion guide optics – when combined with the high-resolution chromatography produced by ACQUITY UPLC System – results in achieving higher sensitivity for molecules, and hence enables the user to quantify molecules with a lower limit of quantitation (LLOQ).

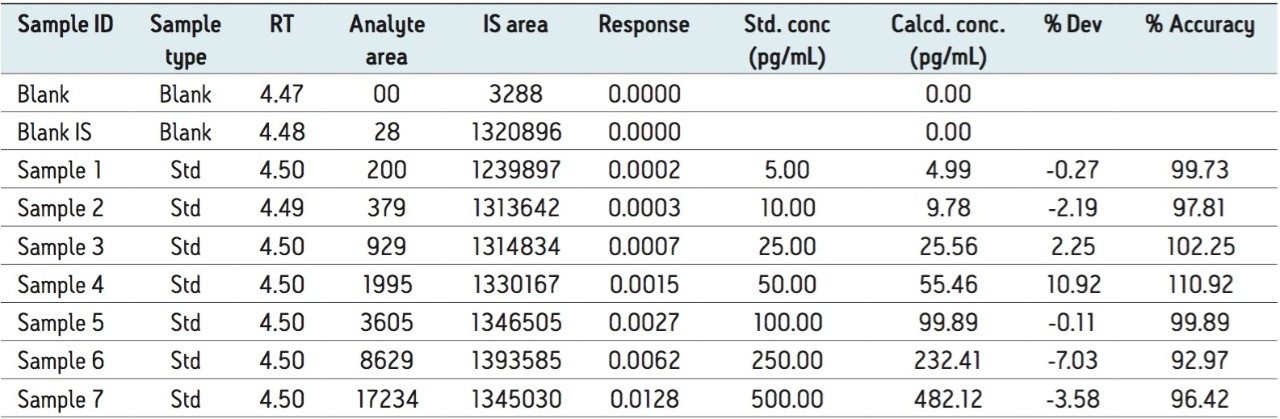
The LC-MS/MS method developed in this application delivered a linear calibration response over the range of 5 to 500 pg/mL with a correlation coefficient of more than 0.99, shown in Table 1. Data displayed in Table 1 also shows the back-calculated concentrations of calibration standards, variation in which is well within the permissible limit (± 15%). Such a result also provides excellent linearity and precision for the method that is used in this application.
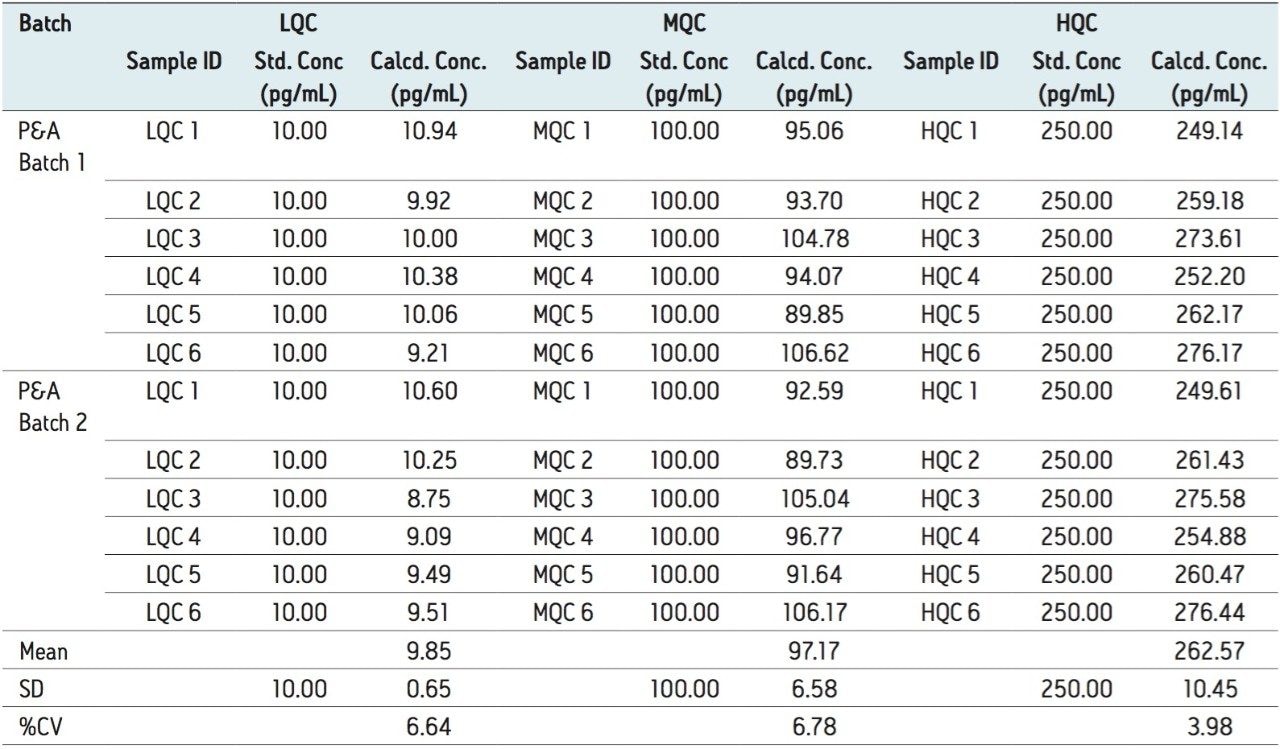
Furthermore, for the global batch studies, two different batches of samples were prepared with three different concentrations (LQC, MQC, and HQC) in each batch. Six samples were prepared for each batch and concentration level. The global %CV of QCs for two PA batches at LQC, MQC, and HQC levels were 6.64%, 6.68%, and 3.98%, respectively, shown in Table 2.
Misoprostol acid is the active metabolite of misoprostol, and is also used as a marker to determine and quantify misoprostol. However, the traditional instrumentation and methodologies failed to provide a sufficiently sensitive method to quantify low concentrations of misoprostol acid in plasma. In this application note, we report a highly sensitive method for the quantitation of misoprostol acid in plasma with an LLOQ of 5 pg/mL. The method showed excellent linearity and precision within the range of 5 to 500 pg/mL. The high sensitivity, precision, and linearity of this method can be attributed to the extraction specificity of Oasis MAX Cartridge, superior resolution of the ACQUITY UPLC System, and outstanding sensitivity of the Xevo TQ-S Mass Spectrometer. The components of Waters Regulated Bioanalysis System Solution provides the user the ability to achieve high sensitivity with their challenging and complicated samples, while addressing several other key challenges such as robustness, reproducibility, precision, and regulatory concerns.
720004415, June 2012