In this application note, a method is converted from analytical ACQUITY (UPC2) to preparative scale for the isolation of impurities in an API mixture.
To minimize the consumption of sample and solvents, there is a benefit in developing separation methods on a small scale and transferring them to a larger scale. The analytical scale method is converted to preparative scale in order to isolate milligram (mg) to gram (g) quantities of purified material. The ability to achieve comparable chromatography between scales when using SFC depends upon several important factors. In this application note, a method is converted from analytical ACQUITY (UPC2) to preparative scale (Prep SFC 150 Mgm) for isolation of an impurity at 0.1% (4-nitrophenol), as it relates to an API (acetaminophen). A cost and time analysis is provided to demonstrate the relationship between column size and throughput when purifying compounds at 19 mm and 30 mm column diameters via SFC.
|
Column: |
Waters, Torus 2-PIC, 130 Å, 5 µm, 4.6 × 100 mm (p/n 186008551) |
|
Injection mode: |
Mixed-stream |
|
Flow rate: |
3.5 mL/min |
|
Co-solvent: |
Methanol |
|
Isocratic: |
80:20 CO2/Co-Solvent |
|
Temp.: |
Ambient |
|
ACQUITY PDA: |
247 nm, 306 nm |
|
Injection volume: |
5 µL |
|
Software: |
Empower 3 Chromatography Data System |
|
Column: |
Waters, Torus 2-PIC, 130 Å, 5 µm, 19 × 100 mm (p/n 186008586) |
|
Flow rate: |
Refer to scale-up calculations (Equation 1 and 2) |
|
Injection mode: |
Modifier-stream |
|
Injection volume: |
500 µL |
|
Temp.: |
35 °C |
|
2489 UV/Vis Detector: |
247 nm, 306 nm |
|
ABPR pressure setting: |
120 bar |
|
Software: |
ChromScope v2.0 |
|
Phase column: |
ACQUITY CORTECS C18 Column, 130 Å, 1.7 µm, 2.1 mm × 150 mm (p/n 186005298) |
|
Flow rate: |
0.50 mL/min |
|
Mobile phase A: |
Water with 0.1% formic acid |
|
Mobile phase B: |
Acetonitrile |
|
Gradient: |
Starting conditions at 20% mobile phase B with a 1 minute hold time, linear increase to 80% mobile phase B over 5 minutes |
|
Column temp: |
40 °C |
|
PDA detector: |
Wavelength 247 nm and 306 nm at 4.8 nm resolution, 3D data scan range 200–400 nm |
|
Injection volume: |
5 µL |
|
Software: |
Empower 3 Chromatography Data System |
A sample solution of API (acetaminophen, Sigma-Aldrich, p/n A3035) and associated impurities, 4-chloroacetanilide and 4-nitrophenol (Sigma-Aldrich, p/n 158631 and p/n 241326) at 0.1% was prepared in a volumetric flask. Solids were fully dissolved in methanol to equal 60 mg/mL (API) and 0.06 mg/mL (impurities), respectively.
Chromatographic method development was performed at analytical scale using ACQUITY UPC2 to separate the API from the impurities. The API (acetaminophen) and impurity (4-chloroacetanilide) were detectable at 247 nm, while the 0.1% impurity of interest (4-nitrophenol) was not adequately detectable at this wavelength. After PDA full spectrum analysis (210–400nm), two wavelengths were selected to provide detection of all compounds present in the mixture.
The flow rate ACQUITY (UPC2) utilized at analytical scale (4.6 mm) was directly scaled to preparative scale (19 mm) using the geometric scale-up formulas.1 Method scale-up was simplified by selecting analytical and preparative columns of the same packing material, length, and particle size. By holding these geometries constant, the column length to particle size ratio (L/dp), important for retention time accuracy, was maintained.
In the UPC2 System, the pump uses volume based flow control (mL/min) for delivering CO2, while in preparative systems, the CO2 pump meters CO2 by mass (g/min). As a result, the actual amount of CO2 delivered differs between the analytical and preparative pumps without compensation for mass flow. These system flow control metering differences must be accounted for by utilizing the density of CO2 (approximately 0.936 mg/mL) and is highly dependent upon system pressure and temperature. The ACQUITY UPC2 analytical flow rate volume flow units were converted to mass flow units (Equation 1) to provide accurate total flow computation at preparative scale (Equation 2).
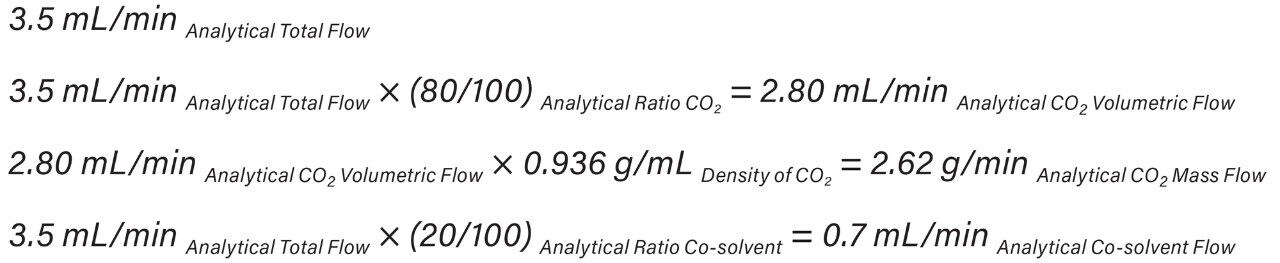
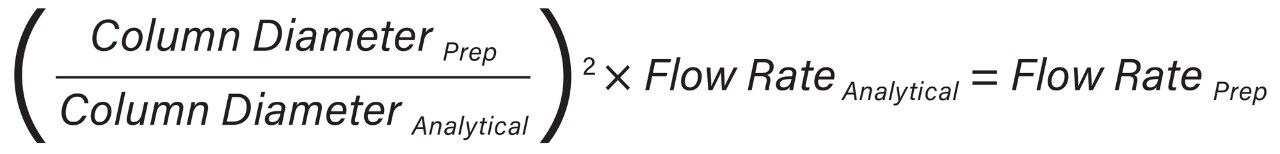


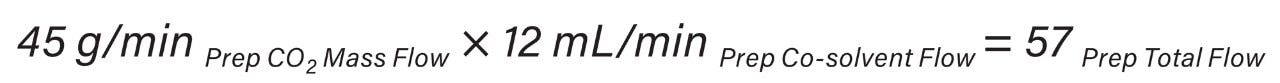
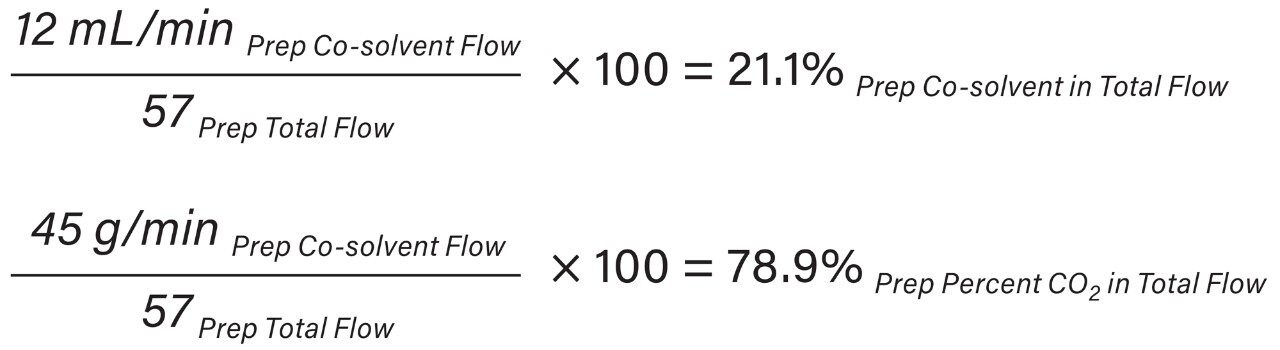
SFC system pressure (run density profile) has an impact on chromatographic separation therefore density profiles must be identical between systems to achieve comparable chromatographic resolution. The system run density profiles were 167 bar at analytical scale and 129 bar at preparative scale (Equation 3).
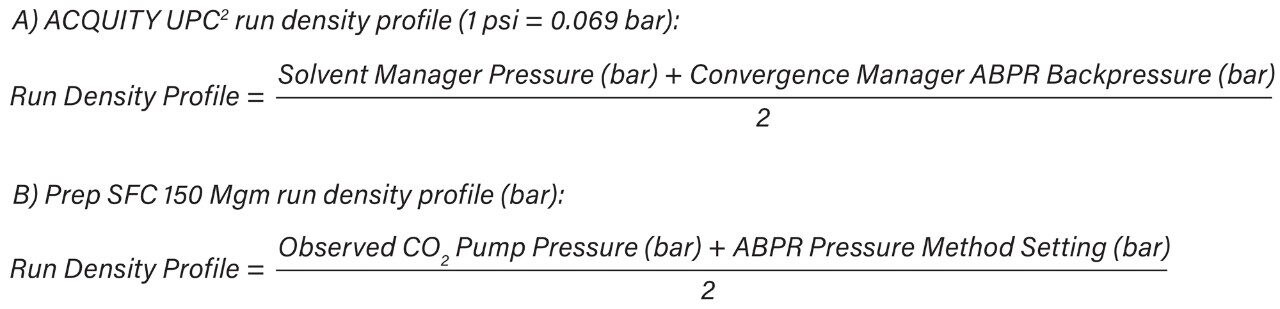
To account for the run density profile differences between the systems during scale-up, modifications to the preparative method were made in order to generate a comparable chromatographic separation. The ABPR Pressure Setting can be adjusted on the Prep SFC 150 Mgm in ChromScope from 120 bar to 158 bar to match the run density profile of ACQUITY UPC2. A complementary approach was employed in this application note. The column temperature used for UPC2 method development was set for ambient temperature, while the CO2 and co-solvent heaters were operated at 35 °C on the Prep SFC 150 Mgm. The increased preparative CO2 temperature resulted in a decrease in the density of CO2 and in the viscosity of the mobile phase. This effect caused a shift in the chromatography, to adequately maintain separation of the target compound and the API without adjustment of the ChromScope software system pressure setting. With this approach, resolution and retention times for the impurity of interest (4-nitrophenol) and the peak eluting prior, were nearly identical when comparing the ACQUITY UPC2 and Prep SFC 150 Mgm separations, as shown in Figure 1.
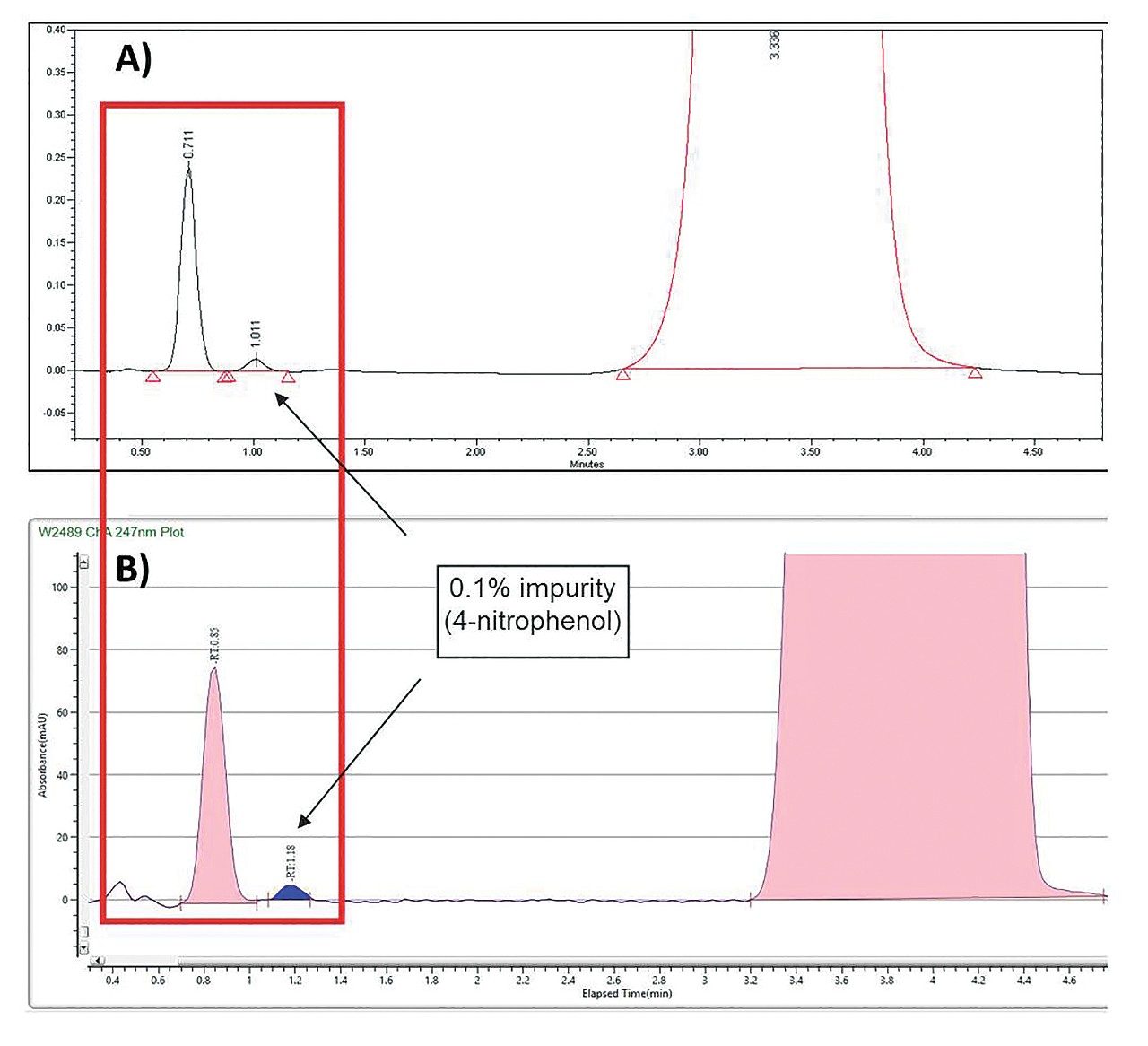
The ACQUITY UPC2 standard configuration employs a mixed-stream injection mode in which the overall mobile phase composition (CO2 plus co-solvent) carries the sample to the column. This mode is not employed for the Prep SFC 150 Mgm (i.e. preparative scale) because strong, polar sample diluents, when injected into the flow path at high volume, can affect the strength of the overall mobile phase. The effect may result in peak distortion and/or a retention time shift. Instead, the Prep SFC 150 Mgm utilizes a patented modifier-stream injection configuration in which the strong, polar sample diluent is mixed with the co-solvent prior to the addition of CO2. This configuration improves peak shape and resolution when using high volume, preparative scale injections.1
For separations intended for scale-up, injection loading studies are sometimes performed at analytical scale to conserve valuable, or volume limited, sample material. The injection load is then mathematically converted to preparative scale via geometric loading calculations.1 With this technique, loading studies at analytical scale require the conversion of the ACQUITY UPC2 System from a mixed-mode to a modifier-stream injection configuration, to result in comparable modes of sample introduction into the flow path.2 In this application note, the sample was not limited or of high value and preparative method development efficiency was highest priority. As a result, injection load determination was performed at the preparative scale via injection of 0.2 mL, 0.5 mL, 1 mL, 1.5 mL, and 1.75 mL. Chromatographic resolution of the target compound was evaluated at each injection volume (data not shown). Because loading studies were performed at preparative scale rather than analytical scale, conversion of the ACQUITY UPC2 from mixed stream injection mode to modified stream injection mode was not required.
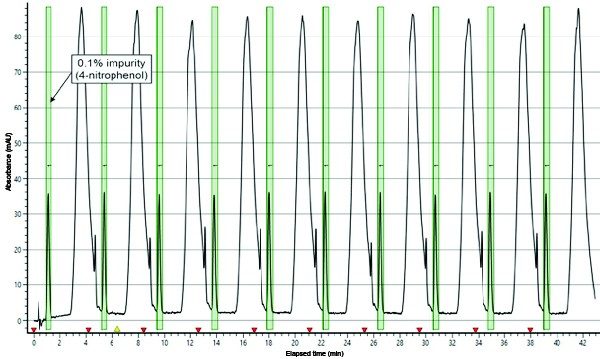
Twenty-five stacked injections of the optimal injection load (0.5 mL) were performed in triplicate on the 19 mm preparative SFC column and collected (Figure 2). Isolates were quantitatively transferred to a 100 mL volumetric flask and diluted to volume with methanol.
Highly concentrated solutions containing low-level target compounds typically require a multi-cycle purification scheme. One approach is to initially isolate the most concentrated component (i.e. API), from a pooled mixture of the low level components (i.e. impurities). A second purification cycle can then be performed on the pooled impurity mixture to further isolate the target compound.
An alternative purification scheme was employed in this application note. Only the target compound was isolated from the sample mixture in the first cycle, followed by a subsequent round of purification to increase the purity of target compound.
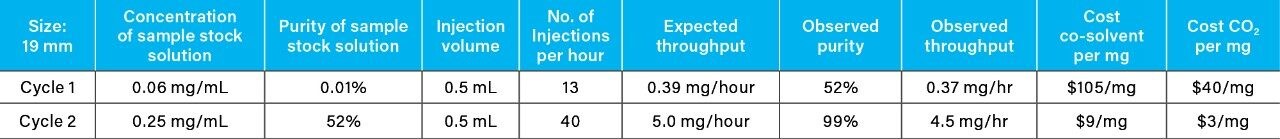
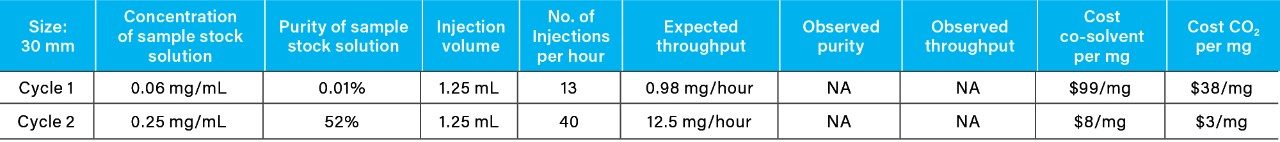
Recovery and purity of the isolates were determined individually by reversed-phase chromatography due to the technique’s high sensitivity and ability to separate compounds orthogonally to SFC. After the initial purification cycle, the average target compound recovery was 92%, and the average purity was 52%. Although the target compound was chromatographically resolved, rapid stacked injections of the concentrated sample solution resulted in low-level cross contamination caused by tailing of the API. This phenomenon is not uncharacteristic when employing rapid preparative scale chromatography for isolation of low-level components from large volume injections of exceedingly concentrated primary sample components, as in the case presented here for the 60 mg/mL API sample solution.
The isolates from the initial purification cycle were dried under nitrogen flow, followed by reconstitution in 10 mL of methanol. They were re-purified via a second cycle using identical parameters as the initial cycle, via triplicate runs of 10 stacked injections. Reversed-phase revealed an increase in purity of the isolate to 99% (Table 1), while the average recovery was maintained at approximately 90%.
Efficiency of the purification described in this application note was increased by utilizing the ChromScope v2.0 software stacking mode for preparative sample injection. This injection mode employs rapid delivery of the sample to the injection loop, in one continuous sample run to efficiently generate the desired isolate. The ChromScope stacked injection mode is especially useful when employed to increase purification efficiency of less concentrated sample solutions, especially those prepared at low concentrations to preserve analyte solubility.
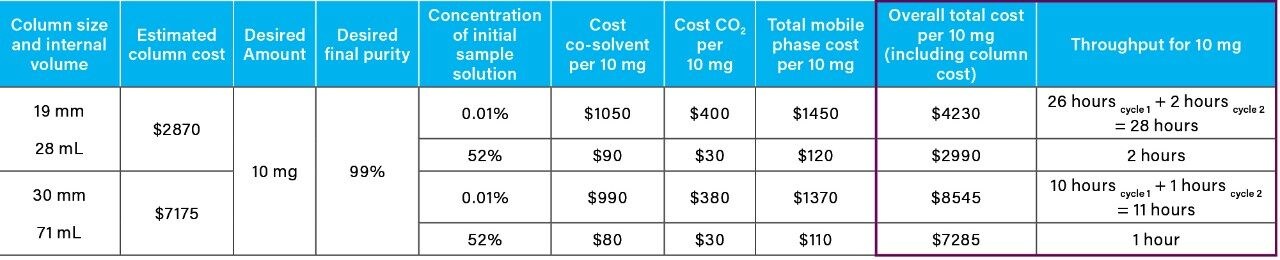
From the recovery and purity of 4-nitrophenol generated using the 19 mm column, geometric scaling and mass load calculations were employed to determine the theoretical cost and purification time for 10 mg using a 30 mm column (Table 2) (Table 3). The cost (CO2 and co-solvent) did not change with column diameter, but the total purification time for 10 mg (throughput) achieved by the larger diameter column (30 mm) showed a significant decrease from 29 hours to 12 hours, when compared to the 19 mm column.
720006436, November 2018