
This application note explains how to utilize the power of Waters ACQUITY UltraPerformance Liquid Chromatography (UPLC), along with fast mass spectrometric acquisition rates, to provide a rapid and selective method to simultaneously analyze 12 water-soluble vitamin compounds in one four-minute run.
As a consequence of global dietary insufficiencies, food manufacturers routinely fortify many food and beverage products to enhance their nutritional value to consumers. Once vitamin compounds have been added to items for consumption, this information should be clearly indicated on the related packaging, in line with strict legal requirements, such as European Regulation (EC) No. 1925/20061 regarding the addition of vitamins and minerals to foods; European Directive 2002/46/EC2 covering dietary supplements; or Title 21 of the U.S. Code of Federal Regulations (CFR) Part 101 – Food Labeling.3
Food manufacturers require rapid, reliable, and cost-effective methods to analyze the nutritional content of their products to ensure that their label claims can be substantiated. Vitamins are often present, even after fortification, at very low levels in challenging matrices, making accurate analyses very demanding.
Currently water-soluble vitamin compounds are analyzed individually, or in small groups, using a wide range of different analytical methods, such as microbiological assays, colorimetric analysis, titrimetric procedures, fluorimetric analysis, and HPLC methodologies.4 This means that analytical laboratories encounter a substantial financial outlay on several different types of instrumentation to facilitate multi-vitamin analysis, as well as a significant investment in personnel time and effort if a series of analyses are to be carried out. The ability to analyze water-soluble vitamin compounds simultaneously in one rapid and selective solution provides businesses with the potential for improved productivity and increased revenue production, resulting from enhanced efficiency, faster sample turnover, and reduced labor and training costs.
This application note describes a quick and selective solution for the simultaneous analysis of 12 water-soluble vitamin compounds commonly used in supplements, or to fortify foods and beverages. This would be particularly suitable for analysts wishing to gain sensitivity, selectivity, and faster sample turn-around time compared with UV detectors for water-soluble vitamin analysis.

Throughout the preparation and analyses, all solutions were protected from exposure to light and stored at <5 °C.
|
LC system: |
ACQUITY UPLC System |
|
Column: |
ACQUITY UPLC HSS T3, 2.1 x 50 mm, 1.8 μm |
|
Column temp: |
40 ˚C |
|
Sample temp: |
4 ˚C |
|
Flow rate: |
0.6 mL/min. |
|
Mobile phase A: |
990 mL water: 10 mL 1M ammonium formate (aq): 1 mL formic acid |
|
Mobile phase B: |
990 mL methanol: 10 mL 1M ammonium formate (aq): 1 mL formic acid |
|
Weak needle wash: |
0.1% formic acid in water |
|
Strong needle wash: |
0.1% formic acid in methanol |
|
Total runtime: |
4 min |
|
Injection volume: |
20 μL, full-loop injection |
|
Needle type: |
Stainless steel (Critical Clean) needle |
|
Time |
Composition |
|---|---|
|
0.00 min |
99% A |
|
2.00 min |
99% A |
|
3.00 min |
45% A |
|
3.10 min |
99% A |
|
4.00 min |
99% A |
|
MS system: |
ACQUITY TQ Detector |
|
Ionization mode: |
ESI + |
|
Capillary voltage: |
1 kV |
|
Desolvation gas: |
Nitrogen, 800 L/Hr, 450 ˚C |
|
Cone gas: |
Nitrogen, 10 L/Hr |
|
Source temp: |
120 ˚C |
|
Acquisition mode: |
Multiple Reaction Monitoring (MRM) |
|
Collision gas: |
Argon at 3.5 x 10-3 mBar |
The advantages of the ACQUITY TQD mass spectrometer compared with the core detectors are the selectivity and sensitivity gains that can be achieved in complex matrices, such as food samples. When the MS is used in MRM mode, specific ions are selected that represent the vitamin of interest. This also means that runtimes may be reduced as peaks do not have to be separated by retention time as is necessary with detection techniques such as UV or fluorescence. The MRM transitions for the compounds under analysis are shown in Table 1, along with additional MS parameters and expected retention times.
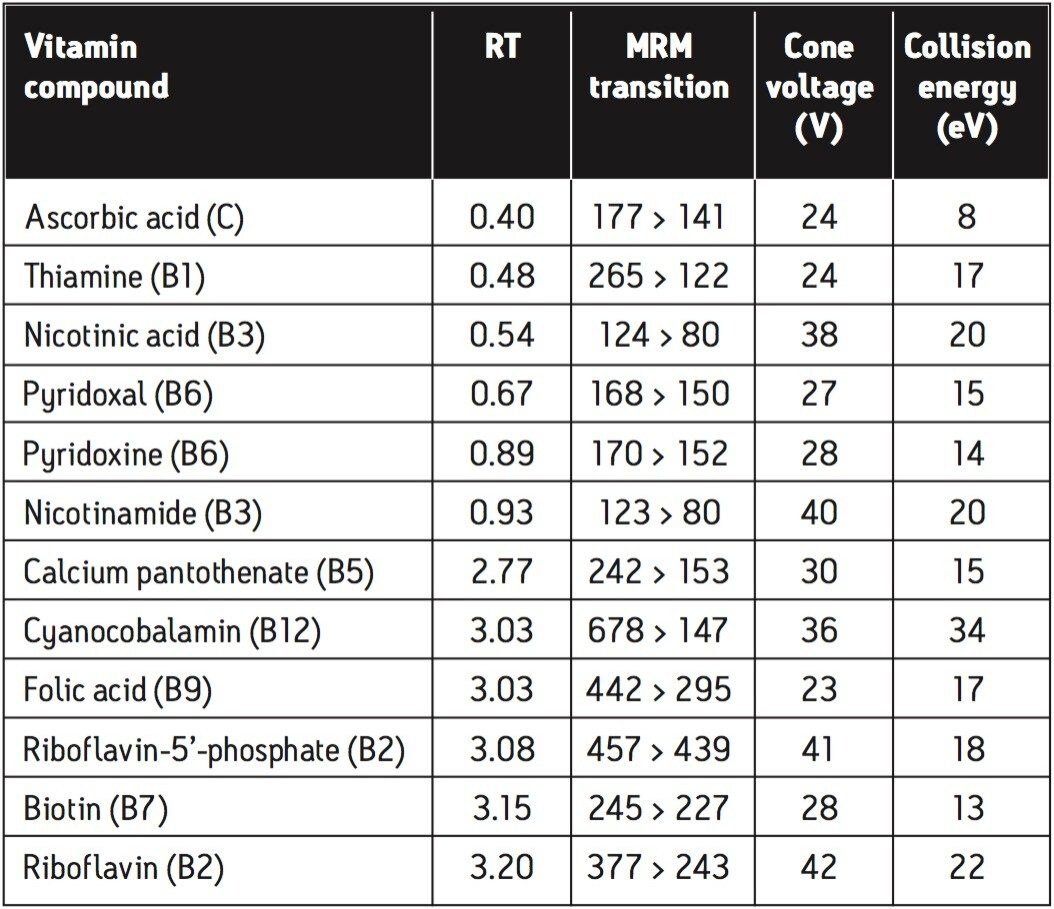
These data were acquired using Waters’ MassLynx Software, v. 4.1.
Incorporated into MassLynx, the IntelliStart Technology automates optimization of MS parameters for the sample, removing the requirement for experienced MS operators to develop the MS method. IntelliStart also monitors the health of the LC-MS system, reducing the time for operator-intensive troubleshooting and upkeep. Automated system check protocols can be scheduled to take place, even outside work hours, and a simple visual indication of the system’s health is provided for the operator.
Data processing was carried out using TargetLynx Application Manager, a quantitation package that enables automated data processing and reporting.
Similar acquisitions and processing can also be carried out using Empower Software.
The analysis of 12 water-soluble vitamin compounds was achieved using ACQUITY UPLC with ACQUITY TQD in MRM mode. The compound-specific transitions in MRM mode enabled a rapid and selective solution to be developed, with the elution of all compounds of interest within 4 minutes.
The extracted ion chromatograms for all 12 water-soluble vitamin solvent standards are shown in Figure 1 for concentrations representative of the lowest levels found in food and beverages. They illustrate that the solution is both rapid and selective, with very good peak shapes for this type of analysis.
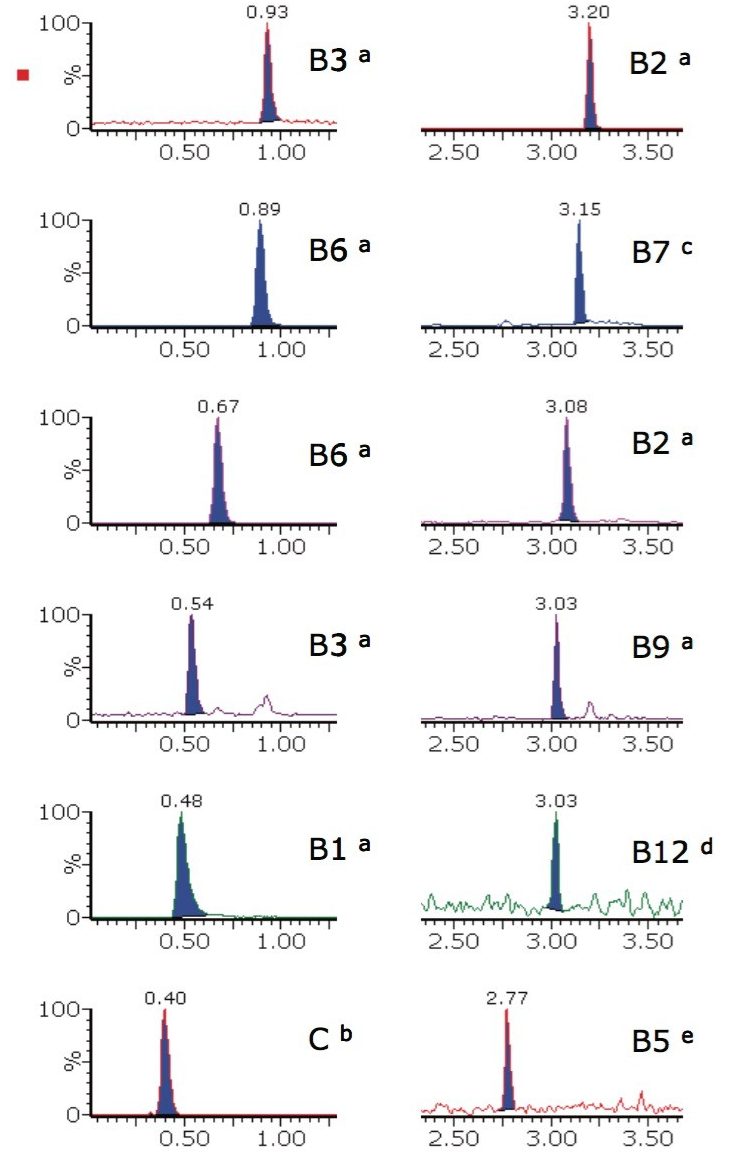
This analysis procedure was tested on two different matrices, however, the chromatographic and MS conditions could be applied to suitably extracted alternative matrices. The analyte list includes more than one form of some of the B-complex vitamins to make it as widely applicable as possible; therefore only the forms used in the tablets or infant formula powder were detected.
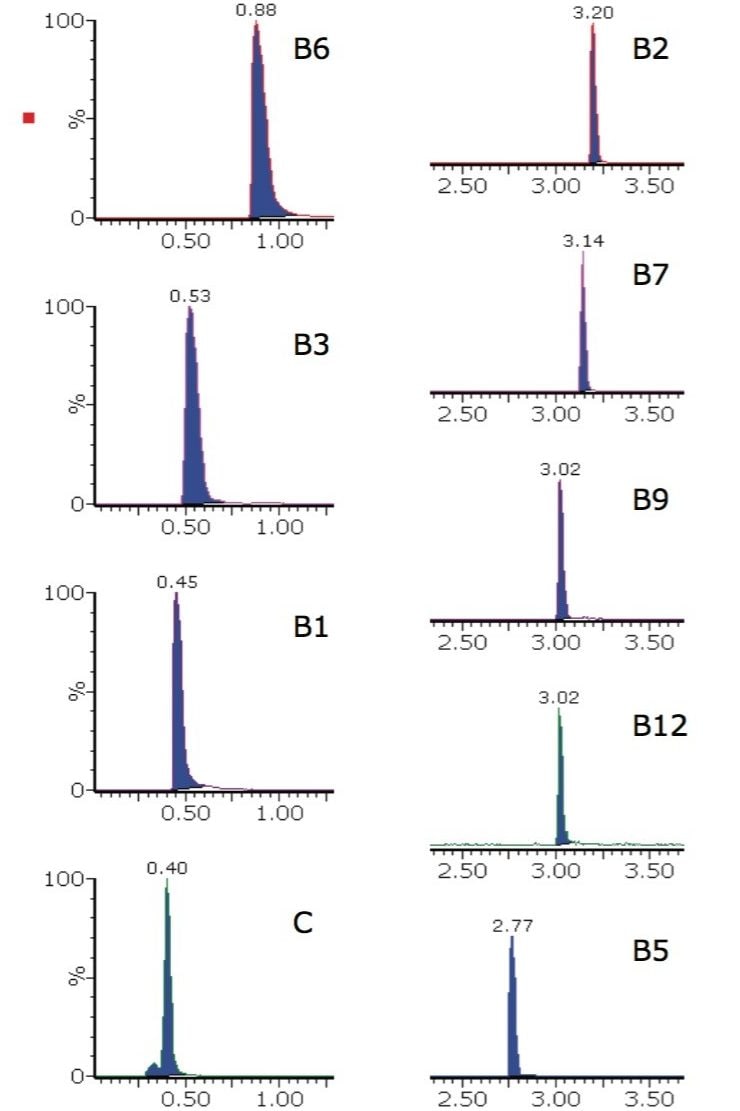
The first matrix examined was vitamin tablets. The extracted ion chromatograms for one brand of dietary supplement vitamin tablet are shown in Figure 2.
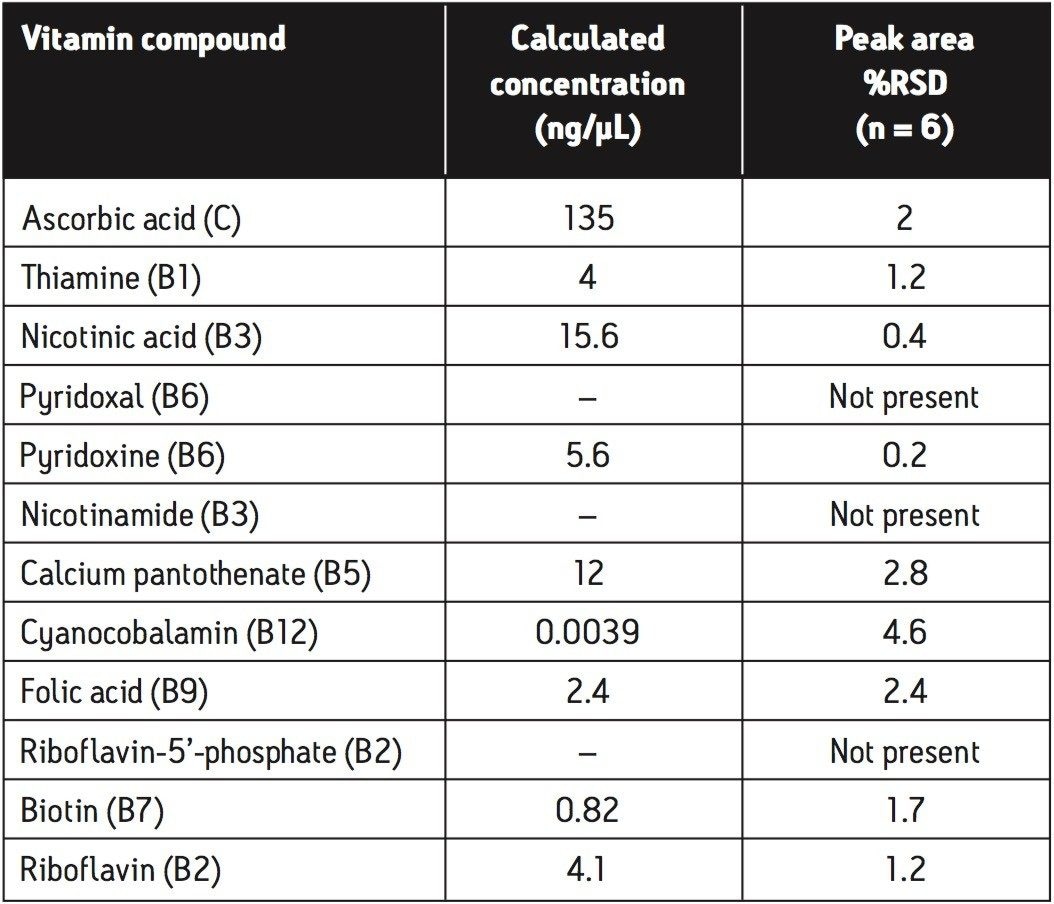
The calculated concentrations and peak area %RSD values, achieved for the vitamin tablet matrix using the analysis described, are shown in Table 2. The %RSD values are an indication of the high level of reproducibility for this analysis when carried out on vitamin tablets.
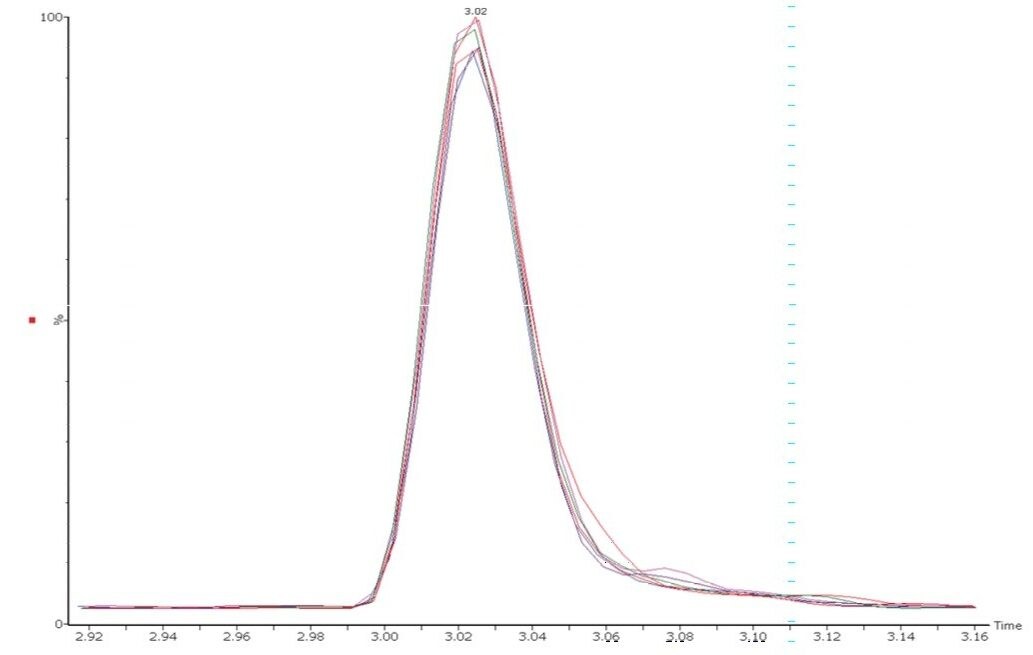
Vitamin B12 is usually the water-soluble vitamin compound fortified at the lowest concentrations in food and beverage commodities. Consequently, the inclusion of B12 in multi-vitamin analysis is often challenging. The solution described here is suitably robust and selective for the analysis of B12 simultaneously with other B-complex vitamins and vitamin C. The peak shapes and retention times for B12 are stable and reproducible over multiple injections, as illustrated in Figure 3.
The analysis conditions were also tested on a more challenging matrix, that of powdered infant formula. The extracted ion chromatograms for one brand of infant formula powder are shown in Figure 4.
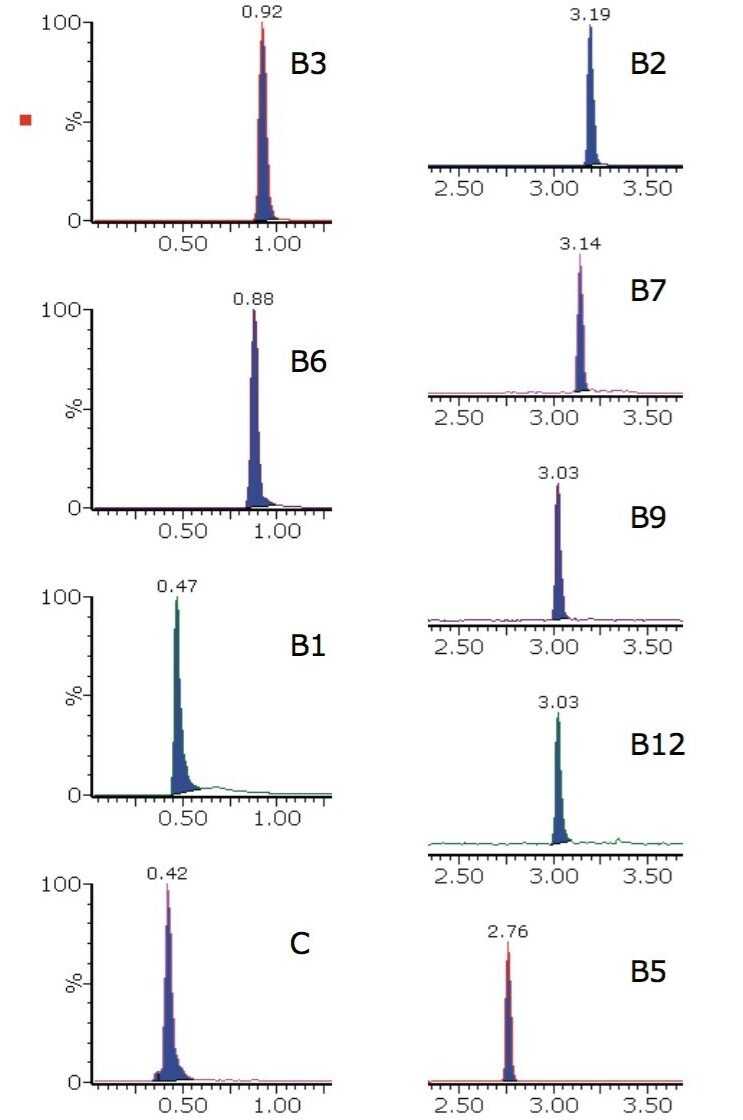
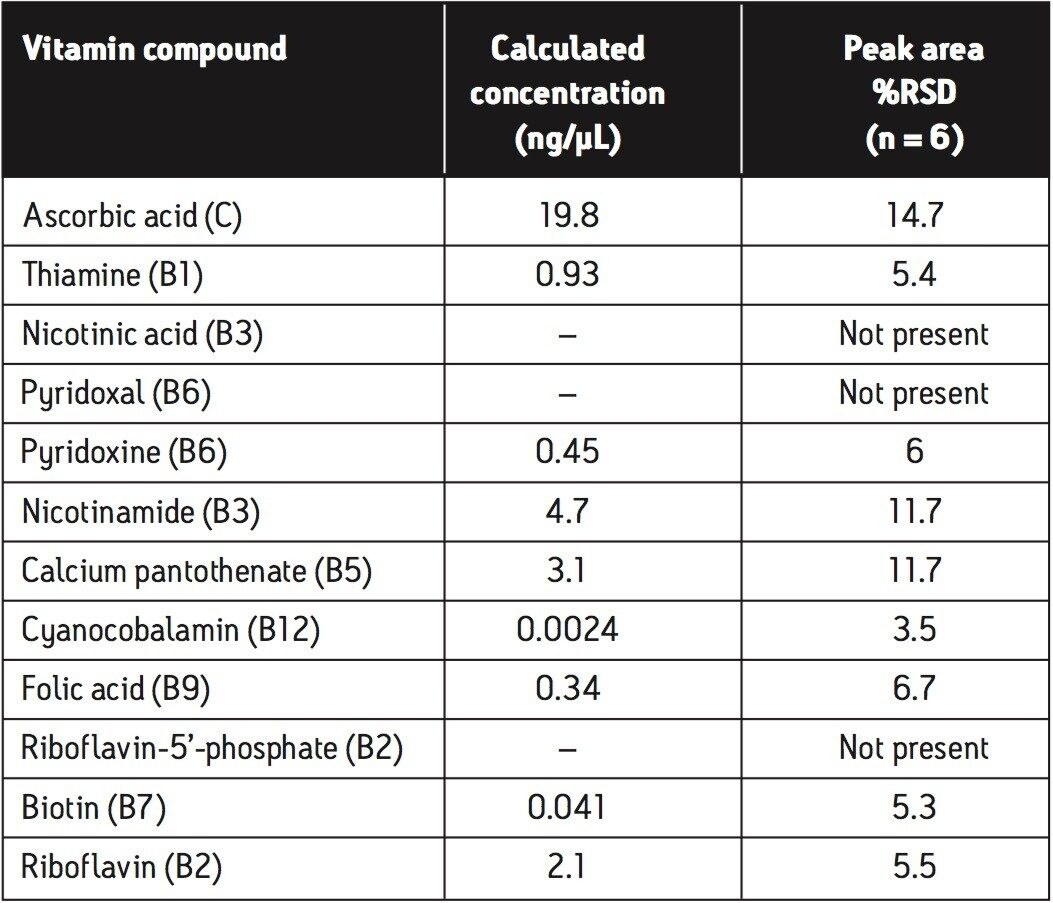
The calculated concentrations and %RSD values for peak area achieved from the infant formula powder matrix, using the analysis procedure described, are shown in Table 3. These %RSD values are obtained following a relatively crude extraction procedure on a very complex matrix. Further improvements in peak area %RSD values may be achieved through more rigorous sample extraction, enrichment, and cleanup procedures prior to data acquisition.
A rapid and selective solution was developed for the simultaneous analysis of 12 water-soluble vitamin compounds in one four-minute run, using the ACQUITY TQD in MRM mode, thus removing the need for separate analysis procedures for each compound. Analysis of dietary supplement vitamin tablets and infant formula powder demonstrates that the solution is applicable to widely differing matrices.
The Waters ACQUITY UPLC with ACQUITY TQD mass spectrometer, as shown in Figure 5, offers:
This system solution, which combines the chromatographic speed of ACQUITY UPLC, with the selectivity of the ACQUITY TQD mass spectrometer, has enabled the amalgamation of 12 different water-soluble vitamin compounds into one single analysis. This offers the revenue-conscious laboratory increased efficiency due to analytical time saving, improved profit through increased sample turn over, and better productivity. In addition, cost savings can be achieved by lowering the use of laboratory consumables, as well as reducing the environmental impact through decreased solvent usage.
This solution provides an ideal basis for a streamlined approach to water-soluble vitamin analysis as it removes the need for multiple methods and offers the analyst the ability to analyze all B-complex vitamins and vitamin C in one rapid and simple procedure.

720003052, June 2009