
The analysis of basic analytes has often been challenging at low pH conditions, particularly when using weak ionic strength additives like formic acid. Columns packed with charged surface materials have demonstrated the ability to minimize undesirable interactions involving basic compounds. This work showcases the improved performance of a column packed with such a stationary phase versus conventional C18 particle technology. Using an Agilent 1290 LC System and a reference standard consisting of basic, neutral, and weakly acidic probes, this superficially porous particle CORTECS C18+ Column provides better peak shapes and improved resolution for basic analytes.
The acid/base character of pharmaceutical compounds can play an important role in how the drug behaves. In particular, the ionization states of these drugs in biological matrices can affect physicochemical properties and pharmacokinetics, which can influence absorption, distribution, metabolism, and excretion (ADME) attributes such as drug permeability, bioavailability, and clearance from the body. In 2014, a study involving over 600 drugs, over 400 of which were basic, demonstrated that around 60% of these basic compounds were ionized at physiological pH. Basic compounds that are charged not only have increased polarity and therefore solubility in water but may also affect the routes of the absorption in the body.1
Analysis using liquid chromatography workflows are important to assess the purity of basic drug targets but can often present problems due to ionization, especially at low pH. When analyzed at low pH, the increased polarity due to the positive charge reduces retention in reversed-phase liquid chromatography (RPLC). In addition, ionic analytes are well known to show characteristics of overloading, i.e., poor peak shape, on reversed-phase columns at lower mass loads than neutral compounds due to secondary interactions with the base particle.2,3
Certain mitigation strategies exist to minimize these interactions including the use of stronger mobile phase additives like trifluoroacetic acid, or even switching to a different stationary phase. Trifluoroacetic acid and other mobile phase additives may improve the peak shape of basic probes but can lead to other issues like reduced MS sensitivity. Changing stationary phases can also be problematic as changes in selectivity can occur, but these can be reduced by selecting an appropriate stationary phase to develop the method.
The work shown here is the separation of the Reversed-Phase QC Reference Material (RP-QCRM) on two different columns packed with superficially porous particles (SPP) and C18 ligands. The first column selected is the CORTECS C18+ 2.7 µm while the second is a superficially porous C18 material made by a different vendor, also with 2.7 µm particles. Peak shape and peak capacity differences will be examined between the two materials.
The Reversed-Phase QC Reference Material (p/n: 186006363) containing a 2 mL solution of 7 compounds (uracil, butyl paraben, naphthalene, propranolol, dipropylphthalate, acenaphthene, and amitriptyline) at pH 7 is provided in a ready to use format requiring no additional sample preparation.
|
LC system: |
Agilent 1290 Infinity I |
|
Detection: |
UV detection at 254 nm |
|
Column(s): |
CORTECS C18+ Column, 90 Å, 2.7 µm, 2.1 x 50 mm (p/n: 186007395) Competitor Superficially Porous C18 Column, 100 Å, 2.7 µm, 2.1 x 50 mm |
|
Column temp.: |
30 °C |
|
Sample temp.: |
Ambient |
|
Injection volume: |
1 µL |
|
Flow rate: |
1.0 mL/min |
|
Mobile phase A: |
0.1% formic acid in water |
|
Mobile phase B: |
0.1% formic acid in acetonitrile |
|
Gradient: |
See Table |
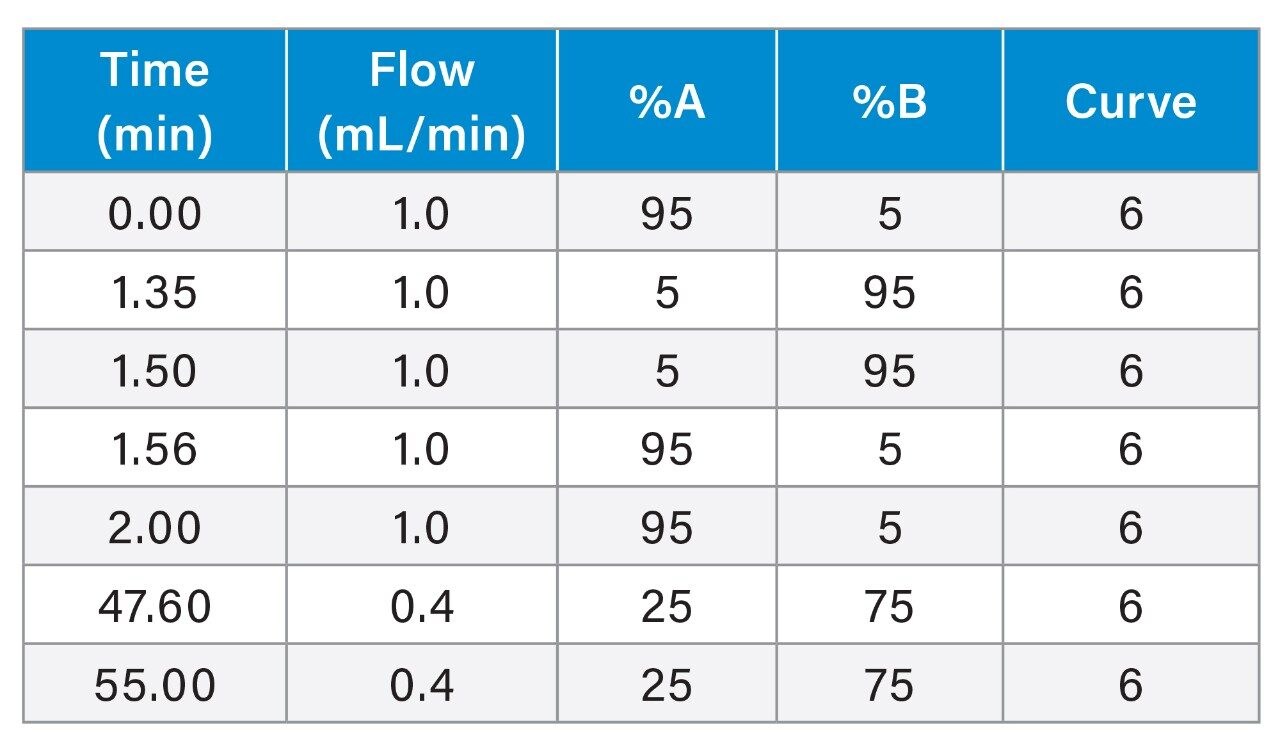
|
Chromatography software: |
Empower 3 Feature Release 5 |
|
Informatics: |
Empower 3 Feature Release 5 |
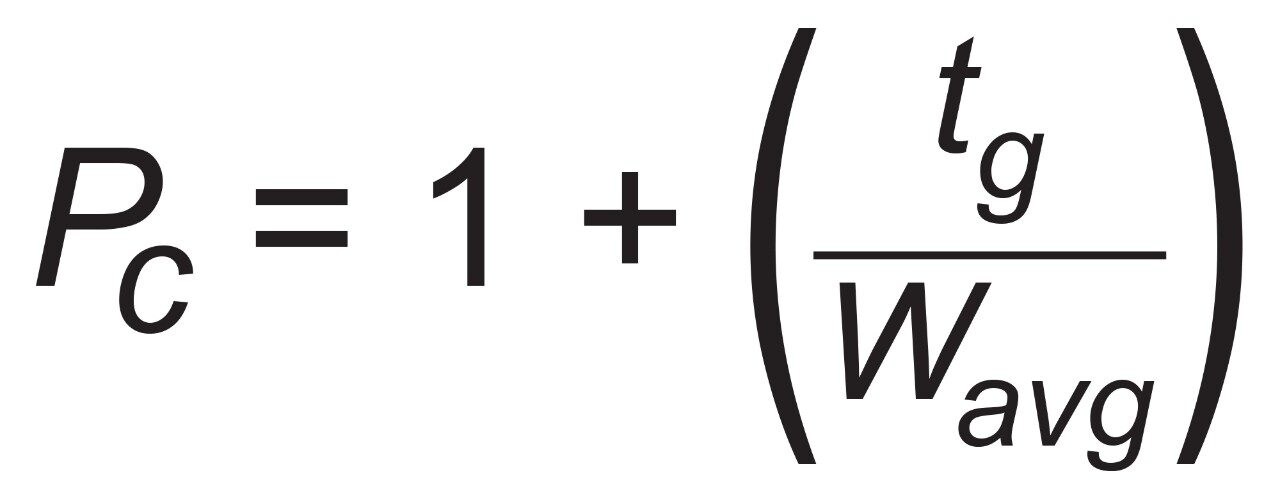
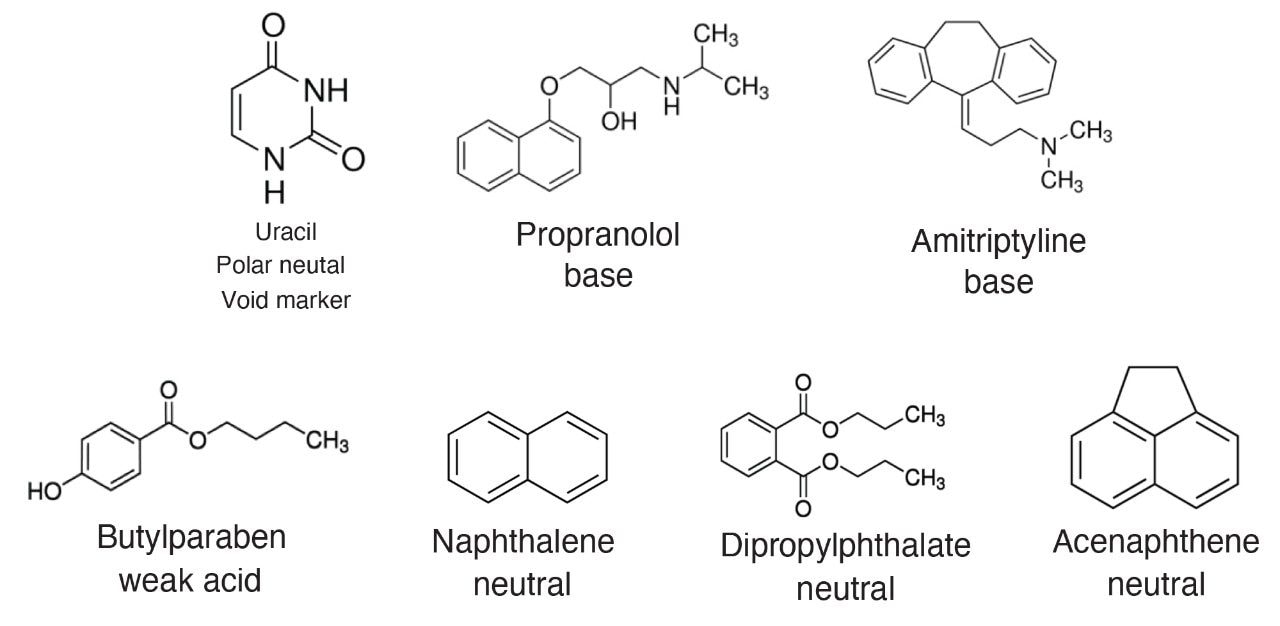
Among the CORTECS family of superficially porous particle (SPP) columns, the CORTECS C18+ material is modified to include a low-level amount of basic functional groups prior to ligand attachment. This same modification is used in columns employing Charged Surface Hybrid (CSH) Technology.2 At low pH, these basic moieties are positively charged and have also been shown to work as a weak anion-exchange functionality for mixed mode separations.4
To illustrate the benefits of the positively charged surface, a CORTECS C18+ Column was used to analyze the Reversed-Phase QC Reference Material (RP-QCRM), a standard containing basic, neutral, and weakly acidic analytes that is designed to measure and monitor system performance. This standard, when run routinely, can also help reduce system downtime by providing crucial data for troubleshooting when an injection fails, regardless of what system is being used. Figure 2 shows the chromatogram of the RP-QCRM separation using the CORTECS C18+ Column versus a competitor superficially porous particle column designed with a conventional C18 stationary phase.
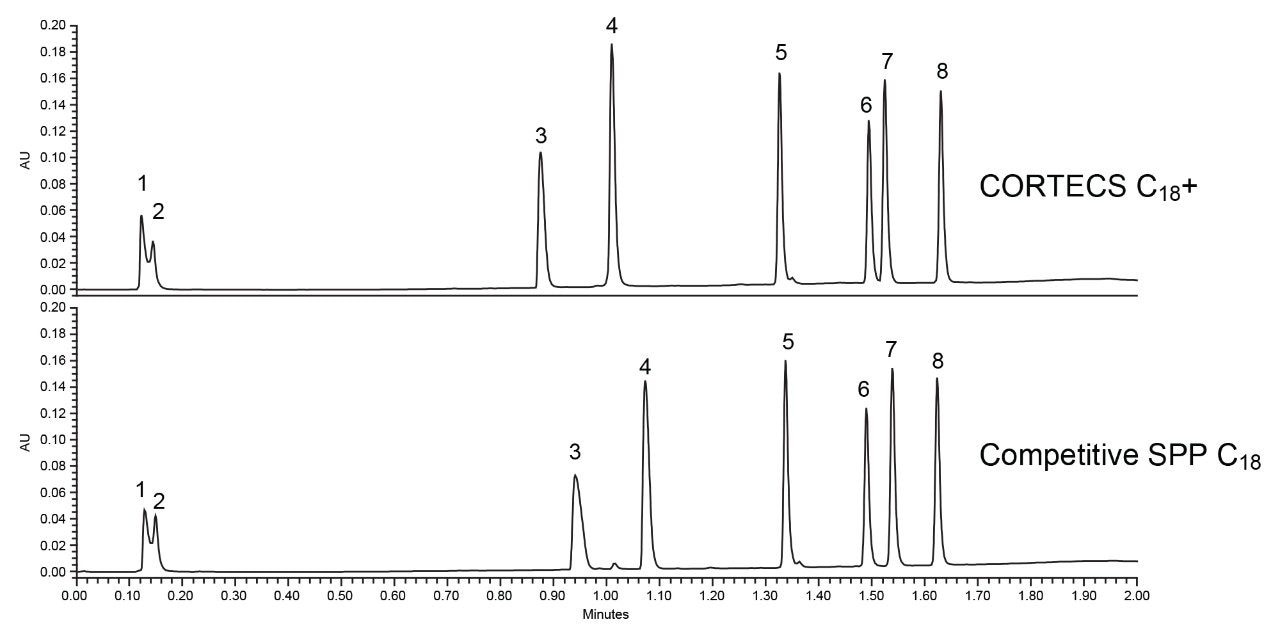
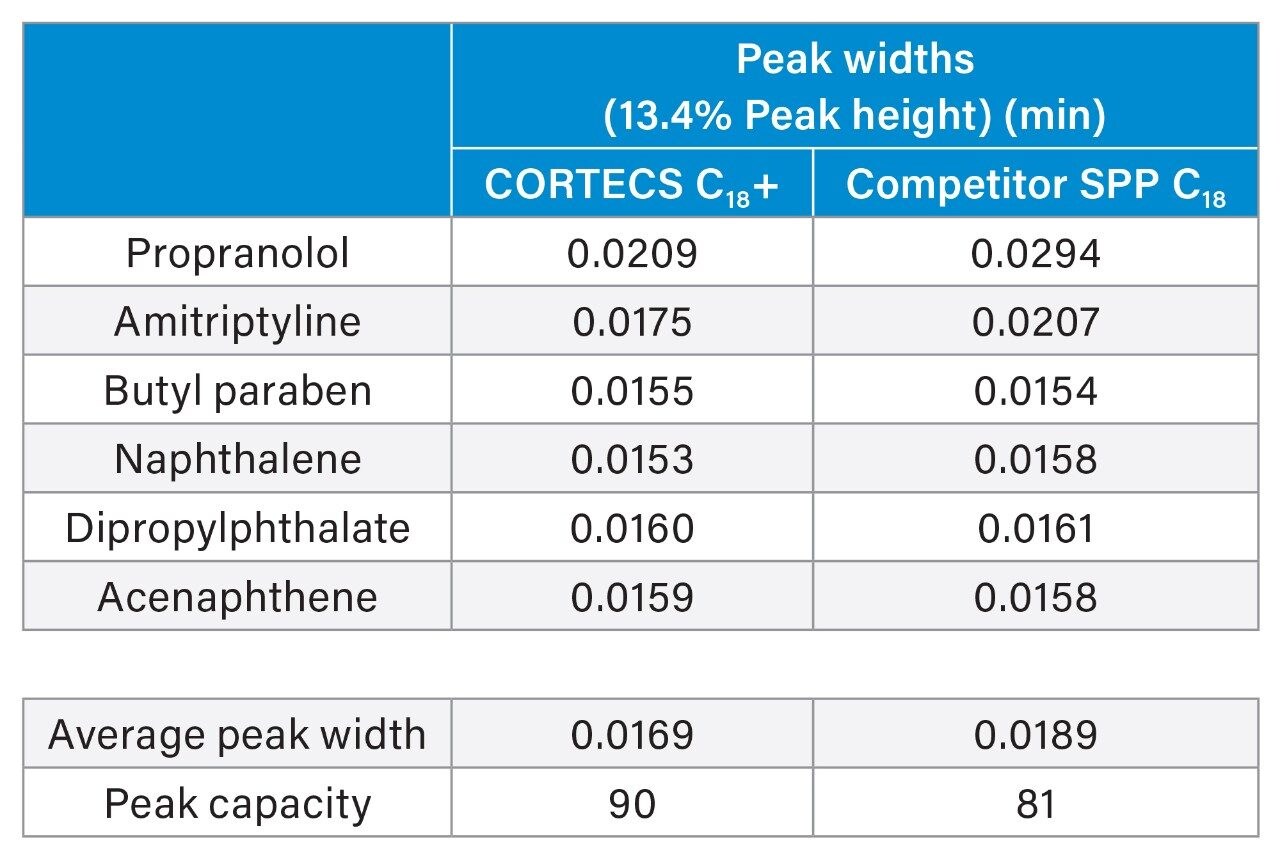
The competitor superficially porous particle C18 column shows comparable results in terms of peak widths for the neutral and weakly acidic probes as well as a marginal difference in selectivity from the CORTECS C18+ Column. The basic probes propranolol and amitriptyline both show poor peak shapes on the competitive column while both have better peak shapes on the CORTECS C18+. As shown in Table 1, narrower peak widths can be achieved for the basic probes when analyzed on the CORTECS C18+, leading to a 10% increase in peak capacity for the separation. The neutral and weakly acidic probes show comparable peak widths between the two columns, indicating that the biggest effect on peak capacity for these two columns is the peak width of the basic probes.
While the slight charge on the stationary phase improve peak shapes, it also leads to slightly less retention as seen in Figure 2 due to an ionic repulsion effect. This effect is not seen for the weakly acidic probe butyl paraben as it is not charged at low pH. For this separation the lower retention of the basic probes does not present an issue. A different assay may require additional consideration to account for this effect. The slight loss of retention is a minor drawback compared to the benefits seen for peak shape of the basic probes. This improvement was achieved without the need for additional method development, or the use of different mobile phase additives.
CORTECS C18+ Columns are packed with superficially porous particles that are modified to include a low-level positive surface charge. This modification mitigates the secondary interactions between basic probes and the stationary phase. This interaction has been known to cause poor peak shapes and poor sample loading especially at low pH with silica based stationary phases. The work shown here illustrates the improvement in peak shape that can be obtained when using CORTECS C18+ Columns versus a non-modified superficially porous particle C18 column. In the case of the reversed-phase QC reference material, the CORTECS C18+ Column yielded a peak capacity 10% higher than the other column tested, due entirely to the peak shape improvements seen for the basic analytes. This improvement was achieved simply by switching the column, without any method development or need to change mobile phases.
720007282, June 2021