For research use only. Not for use in diagnostic procedures.
In this application note, we demonstrate the utility of a high throughput, multi-omic workflow (proteomics and lipidomics) for a plasma-based sample set, consisting of healthy controls and breast cancer individuals. Resulting lipid and protein DIA datasets have been integrated and interrogated to provide insights into the biological pathway and associated networks connected to the pathogenesis of the disease.
Breast cancer is one of the most common cancers diagnosed in women and the second leading cause of death after lung cancer. Breast cancer types consist of two main categories, termed in situ and invasive, with invasive being the most common (81% of cases).1 Omic-based strategies have previously been shown to provide insight into the biological mechanisms of various cancer types, including breast-focused studies.2,3
Rapid profiling methods for discovery-based analyses have previously been demonstrated for omic-based studies,4,5 whereby microbore chromatography is configured with data independent acquisition (DIA) schemas to allow for increased sample throughput, while retaining highly confident identifications.
Here, we demonstrate the utility of a high throughput, multi-omic workflow (proteomics and lipidomics) for a plasma-based sample set, consisting of healthy controls and breast cancer individuals. Resulting lipid and protein DIA datasets have been integrated and interrogated to provide insights into the biological pathway and associated networks connected to the pathogenesis of the disease.
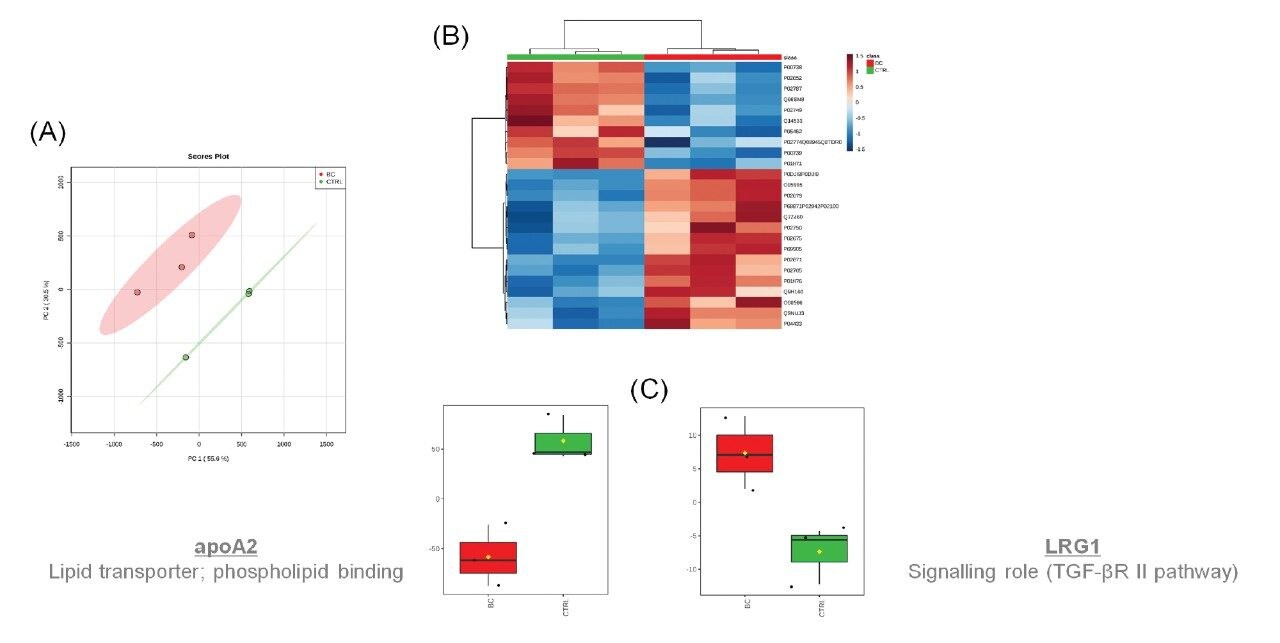
Plasma samples originating from 26 individuals were used for the study. These comprised of healthy controls (n=6) and breast cancer (n=20) diagnosed individuals(Figure 1).
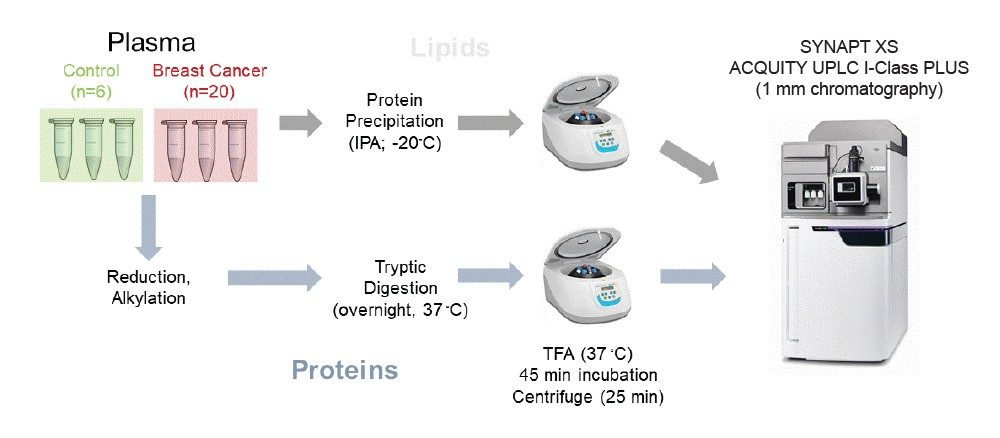
A simple sample preparation procedure was adopted using protein precipitation with pre-cooled isopropanol (1:5 plasma:IPA). Samples were mixed for one minute and placed at -20 °C overnight. The extracted samples were centrifuged at a maximum of 10,300 g for 10 minutes at 4 °C before transferring the supernatant to Waters Total Recovery UPLC Vials (p/n: 186005669CV) for LC-MS analysis. Prepared samples were analyzed in triplicate for both ionization modes.
Undepleted plasma were prepared with 1% RapiGest SF Surfactant prior to reduction, alkylation, and overnight digestion with trypsin.
|
LC system: |
ACQUITY UPLC I-Class PLUS |
|
Column(s): |
ACQUITY UPLC BEH C8 1.0 × 50 mm, 1.7 μm |
|
Column temp.: |
55 °C |
|
Flow rate: |
0.25 mL/min |
|
Mobile phase: |
Water:isopropanol:acetonitrile (50:25:25)/5 mM ammonium acetate/0.05% acetic acid (A) and isopropanol:cetonitrile (50:50)/5 mM ammonium acetate/0.05% acetic acid (B) |
|
Gradient: |
1% to 90% B |
|
Run time: |
3 min |
|
Injection volume: |
0.2 μL |
|
LC system: |
ACQUITY UPLC I-Class PLUS |
|
Column(s): |
ACQUITY UPLC CSH 1.0 × 100 mm, 1.7 μm |
|
Column temp.: |
45 °C |
|
Flow rate: |
50 μL/min |
|
Mobile phase: |
Water + 0.1% formic acid (A) and acetonitrile + 0.1% formic acid (B) |
|
Gradient: |
1% to 40% B |
|
Run time: |
15 min |
|
Injection volume: |
2 μL |
|
MS system: |
SYNAPT XS |
|
Ionization mode: |
ESI (+/-) |
|
Capillary voltage: |
2.8 kV (+) 1.9 kV (-) |
|
Acquisition mode: |
HDMSE |
|
Acquisition rate: |
Low and elevated energy functions at 0.1 seconds |
|
Collision energy: |
5 eV (low energy function); 20–45 eV linear collision energy ramp (elevated energy function) |
|
Source temp.: |
120 °C |
|
Desolvation temp.: |
500 °C |
|
Cone gas flow: |
50 L/hr |
|
Desolvation flow: |
800 L/hr |
|
MS system: |
SYNAPT XS |
|
Ionization mode: |
ESI (+) |
|
Capillary voltage: |
2.1 kV |
|
Acquisition mode: |
SONAR |
|
Acquisition rate: |
Low and elevated energy functions at 0.5 seconds |
|
Collision energy: |
5 eV (low energy function); 20–45 eV linear collision energy ramp (elevated energy function) |
|
Source temp.: |
120 °C |
|
Desolvation temp.: |
500 °C |
|
Cone gas flow: |
50 L/hr |
|
Desolvation flow: |
600 L/hr |
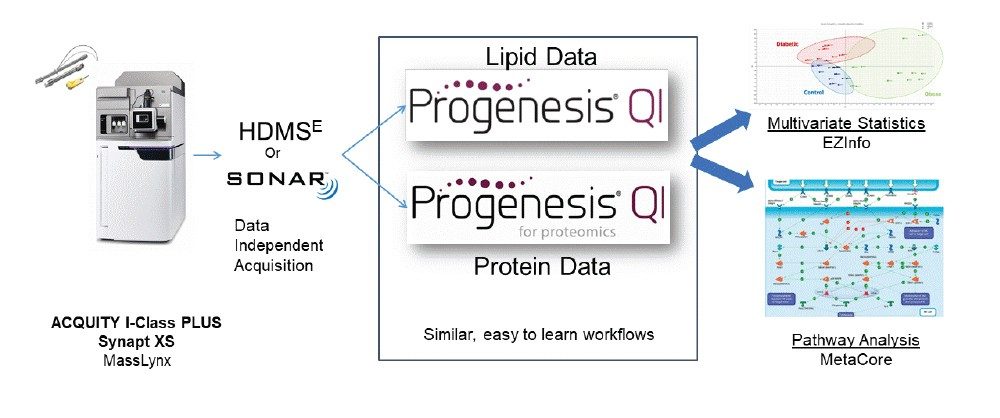
Progenesis QI and Progenesis QI for Proteomics were used for processing the lipidomic and proteomic datasets respectively. In both cases, data were aligned and normalized to provide label-free quantification. Lipid identifications were provided by searching against the LIPID MAPS database. Resulting identifications were further curated using the Lipid Reporter Tool.6 Protein/peptide identifications were returned when searched against the UniProt human database, containing only curated entries. Carbamidomethyl (C) and oxidation (M) were considered as fixed and variable modifications respectively, with a 1% FDR applied. Statistical analysis in all cases was conducted using a combination of EZinfo (Umetrics, Umeå, Sweden) and MetaboAnalyst (University of Alberta).7 The curated lipid and protein results were directly outputted from Progenesis for pathway and network mapping using Metacore, a Cortellis solution (Clarivate Analytics, London, UK) (Figure 2).
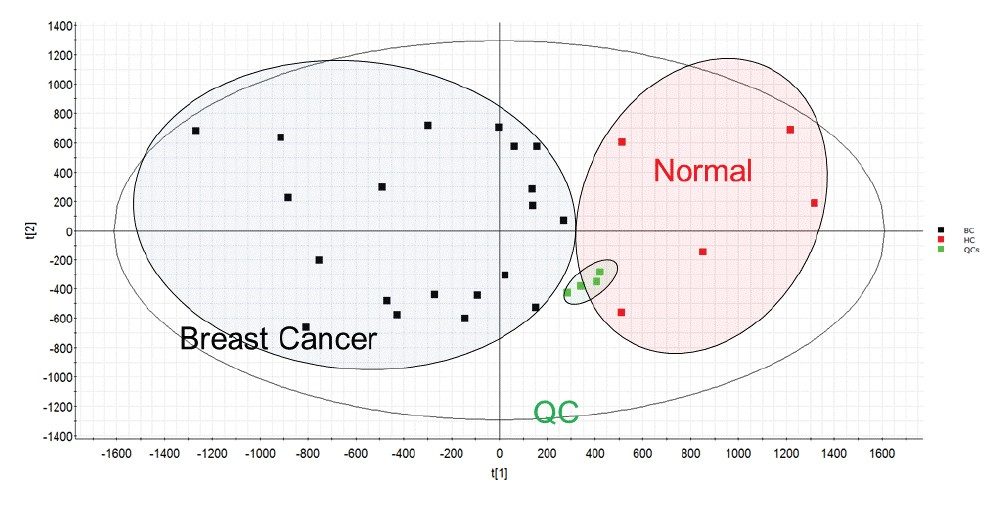
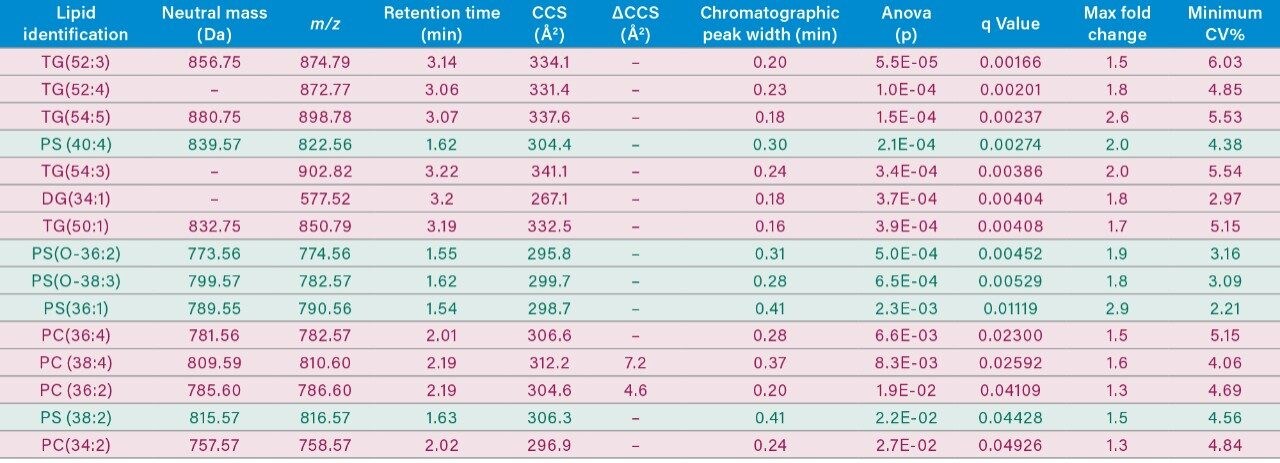
Extracts resulting from control and breast cancer samples were analyzed using a high throughput approach combined with DIA strategies. Ion mobility (IM)-based DIA was utilized for lipidomic analyses, while SONAR was employed for proteomics. Rapid profiling of the lipidome combined with multivariate statistical approaches highlighted key differences between the control and breast cancer groups (Figure 3). Orthogonal partial least squares (OPLS-DA) indicated phospholipids and neutral lipid species (i.e., triglycerides) as being the dominant drivers for separation between the two cohorts (Table 1).
Complementary proteomic analysis of the plasma extracts identified 170 curated protein identifications and demonstrated a dynamic range >3 orders. Furthermore, when comparing the proteome using multivariate statistical approaches, significant differences between the control and breast cancer cohorts were revealed. Unsupervised principal component analysis (PCA) clearly shows this separation between groups (Figure 4A), while the most significant proteins contributing towards the variance are presented as a heatmap (Figure 4B). ApoA2 is under-expressed in those subjects with breast cancer. ApoA2 is involved in the lipid transportation and, more specifically, phospholipid binding, corresponding with the increased levels of PS lipids observed from the lipidomic analyses. Previous studies have also shown correlation between the role of ApoA2 in other cancer types, such as pancreatic.8 LRG1, a protein involved in signalling within the TGF pathway, is shown to be over-expressed for breast cancer and has previously been reported as being involved in ovarian cancer (Figure 4C).9
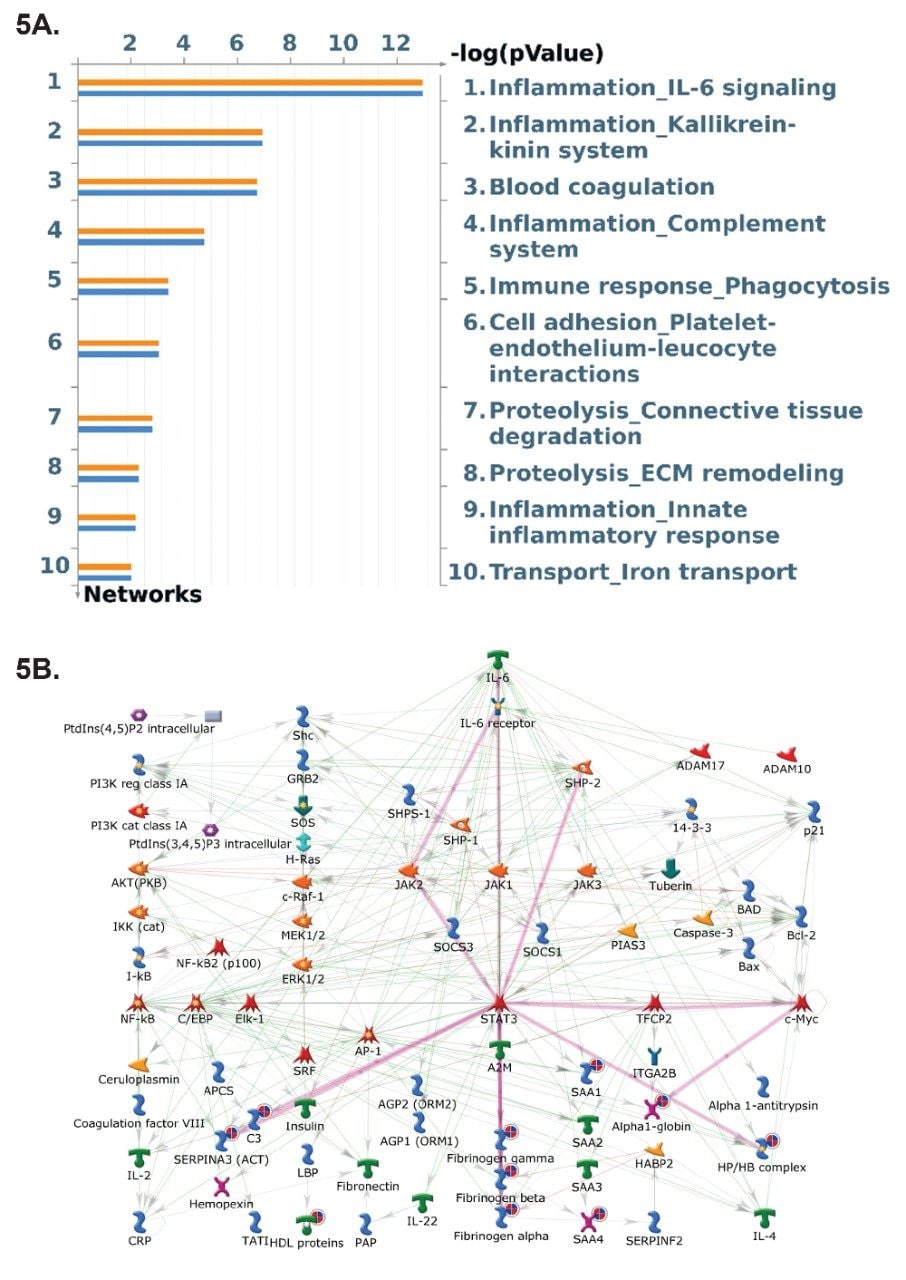
In order to provide biological context, both the proteomic and lipidomic datasets were subjected to pathway analysis using Metacore, a Cortellis solution. Streamlined integration of Progenesis with Metacore enables multi-omic datasets to be readily interrogated for both pathway and network analyses. Several pathways resulting from enrichment analysis were highlighted as being statistically significant based on the contribution from both lipids and proteins (Figure 5) with inflammation IL-6 signalling being the highest ranking. The relationship between complexes identified as a result of enrichment analysis can be highlighted through network analysis. Focusing on the IL-6 signalling pathway, the signal transducer and activator of transcription 3 (STAT3) is responsible for mediating the transcriptional programs downstream of several cytokine, growth factor, and oncogenic stimuli. Its expression and activity are consistently linked to cellular transformation, as well as tumor initiation and progression. All links/ associations between the identified proteins are shown to interact through STAT3 (Figure 5).
720006815, March 2020