With the introduction of BioResolve SEC mAb Columns came a newly optimized shipping and storage solvent. Five different long-term size-exclusion chromatography (SEC) column storage solvents were investigated using BioResolve SEC mAb Columns and the Waters mAb Size Variant Standard. Column performance was monitored after one, two, and four months of storage in one of the five tested solutions. Each column’s performance after storage was compared to its initial performance. Data directly relevant to the routinely monitored critical quality attribute of protein aggregates and self-associated forms (high molecular weight species, HMWS) are shown as well as data for the characterization of the ~100 kDa low molecular weight species (LMWS).
The results of this investigation indicate that storage solvents containing a buffer and salt maintained the initial column performance better than similar aqueous organic solvents free of buffer and salt. Concerns over storing columns in chloride containing buffers are addressed.
It is well known that the mobile phases used for the characterization of native peptides and proteins in SEC are capable of growing microorganisms which can “infect” columns and lead to degradation of a column’s resolving power. Furthermore, it is known that these columns infected with microorganisms produce contaminated fractions. What might not be appreciated is the fact that the mobile phases used for the characterization of native peptides and proteins are well within the pH and salt ranges that enhance bacterial growth.1,2,3
Most SEC column manufacturers use sodium azide as the bacteriostatic agent for the shipping and storage of SEC columns. Concentrations between 0.02–0.05% are typically used with the highest recommended concentration of 0.1%. Sodium azide at a concentration of 0.05% has been found to be effective as a bactericide for many gram-negative bacterial.4 Gram-positive bacteria are more resistant to sodium azide and have been found to grow in media containing 1% sodium azide.5 The content of the cited papers and others6,7 indicate that there is no silver bullet for preventing microbial growth that would be suitable under SEC conditions. Due to a bacteria’s capacity for rapid change via horizontal DNA transfers (plasmids), bacteria find ways to adapt to stressors. As one of the earliest examples of this, just four years after the scaled-up production of penicillin (1947), the first strains of penicillin resistant Staphylococcus aureus were found. The best recourse against damage to SEC columns due to microbial “infection” is prevention. Unlike other modes of column fouling, microbial growth can continue even when the stored column is not in use if it does not contain a bacteriostatic or cidal reagent.
At the time of this writing, Waters Corporation declines/prohibits the use of sodium azide in any of its manufacturing facilities because of the risks associated with its use. Sodium azide is a highly water soluble, inorganic salt that is very acutely toxic, RTECS #VY8050000.8 Even small amounts, if swallowed, can be fatal9 and no known antidotes have been found.10 Mixing sodium azide with acid produces the highly toxic hydrazoic gas and contact with copper, lead, brass, or solder in plumbing systems can lead to the formation and accumulation of explosive metal azides.11
For these reasons we have chosen to use 20% methanol/80% water or 10% acetonitrile (ACN) in 25 mM sodium phosphate (Na-PO4) pH 7, 100 mM potassium chloride (KCl) as the bacteriostatic solutions for the elimination/minimization of microbial growth during SEC column storage. Based on our historical use of these solutions12 we believe they are as effective as sodium azide in protecting SEC columns from microbial growth. Searches for organic solvent-tolerant (OST) bacteria, useful for remediation purposes, indicate that OST bacteria are difficult to find. The successful identification of an acetonitrile tolerant strain found that 10% ACN killed the bacteria when in its initial growth phase.6
The goal of SEC column storage protocols is to maintain existing chromatographic performance of a column during short term and/or long term intervals of inactivity. Common issues encountered after storage are changes in protein retention times, poor recovery of HMWS, and/or loss of the resolving power for the column. The focus of this investigation is to provide experimental support for the choice of our new shipping/storage solvent: 10% acetonitrile (ACN) in 25 mM Na-PO4 pH 7, 100 mM KCl.
Changes in protein retention times are typically minor for SEC columns in buffers with pH < 7. Other common issues associated with column storage, although not desirable, can typically be mitigated with injections of protein samples such as Waters BEH200 SEC Protein Standard Mix (p/n: 186006518) to re-condition or “re-passivate” the column. The most significant factor contributing to the catastrophic failure of a column during storage is microbial growth. This failure mode is not directly addressed in this application note, but based on historical use in our laboratories, low concentrations of organic solvents effectively mitigate the risk of microbial growth in SEC columns free of gross contamination. The goal of this application note is to review the impact of the long-term storage solvents on the performance of BioResolve SEC mAb Columns after storage periods of one to four months.
Before proceeding to the long-term storage data, the following provides general guidance for maintaining column performance during short-term storage:
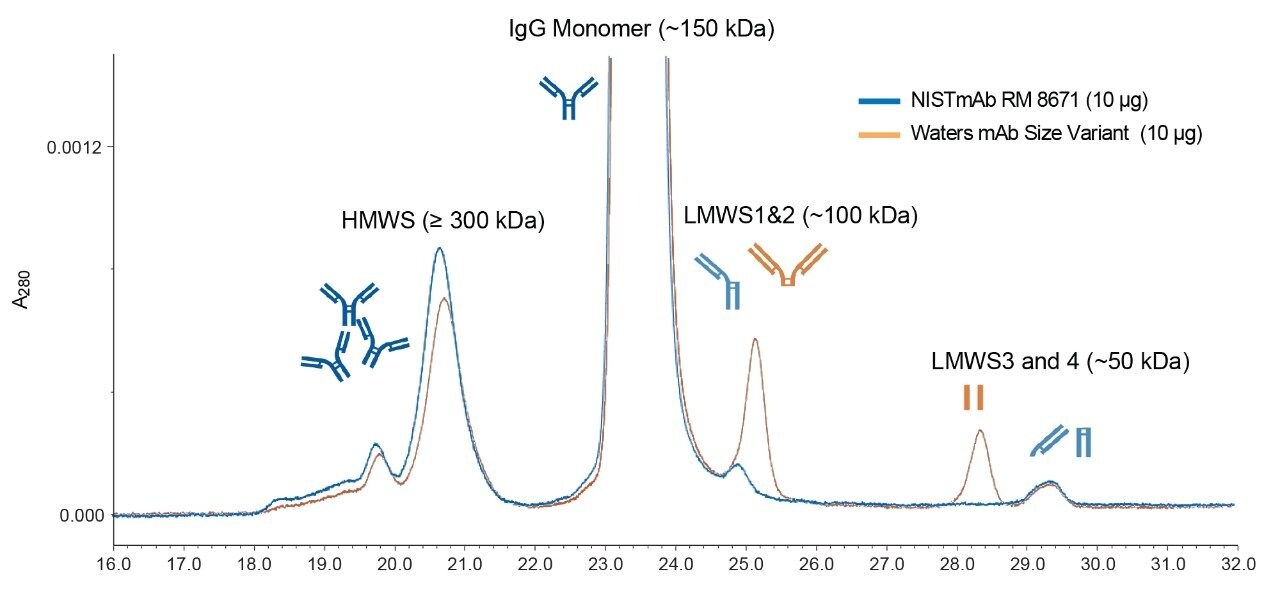
Waters mAb Size Variant Standard (p/n: 186009429) contains 160 µg of stabilized and lyophilized NISTmAb RM8671 which has been supplemented with 2 µg of non-reduced IdeS (Fabricator) digested NISTmAb fragments (F(ab')2 and (Fc/2)2). The lyophilized contents of each vial were solubilized using 70 µL of Milli-Q water. More information on the Waters mAb Size Variant Standard can be found on waters.com, search for 720006811EN.
|
Systems: |
ACQUITY UPLC H-Class Bio |
|
Detectors: |
Tunable Ultraviolet (TUV) with a 5 mm Ti Flow Cell for H-Class Bio |
|
Detection: |
280 nm, 10 Hz, fast filter |
|
Vials: |
Max Recovery Sample Vials (p/n: 186000327C) |
|
Column(s): |
BioResolve SEC mAb, 200 Å, 2.5 µm, 4.6 x 150 mm (p/n: 176004592*) *Includes column and one complimentary vial of mAb Size Variant Standard |
|
Column temp.: |
35 °C Active preheater CH-A (H-Class) |
|
Sample temp.: |
8 °C |
|
Sample: |
2.28 mg/mL Waters mAb Size Variant Standard |
|
Injection volume: |
1.8 µL |
|
Flow rate: |
0.200 mL/min |
|
Seal wash: |
10% HPLC-grade methanol/90% 18.2 MΩ water v/v (seal wash interval set to 0.5 min) |
|
Sample manager washes: |
18.2 MΩ water |
|
Mobile phase A: |
50 mM Sodium phosphate pH 7.0, 200 mM KCl |
|
Mobile phase B and C: |
18.2 MΩ water |
|
Mobile phase D: |
10% Acetonitrile/90% 25 mM sodium phosphate pH 7.0 + 100 mM potassium chloride |
|
Syringe draw rate: |
30 µL/min |
|
Needle placement: |
1.0 mm |
|
Air gaps: |
None |
|
Data channels: |
System pressure, room temperature |
|
Mobile phase A: |
Prepare by mixing 2.66 g of anhydrous dibasic sodium phosphate, 4.36 g of monobasic potassium phosphate mono hydrate, and 14.91 g of potassium chloride per L of water followed by filtration using sterile 0.2 µm nylon filter units (filtered mobile phase pH 6.9) |
|
Chromatography software: |
Empower 3, FR 3.0 |
There is very little literature comparing the effects of various column storage solvents on the performance of SEC columns. Only one paper could be found which compared 100% methanol, 10% methanol, and 0.001% sodium azide in water as storage solvents.14 The 100% methanol solvent was found to remove peptides that had previously been used to condition the examined SEC columns.14 Support for the use of buffers containing sodium azide and low organic/water solvent solutions can be found in most SEC column manufacturer’s instruction manuals. Unique to the shipping/storage solvent recommended for BioResolve SEC mAb Columns is the addition of a buffer plus salt to a 10% acetonitrile solution.
The present study explores the effects of 10% acetonitrile in water or in combinations of buffers with and without salt. Concerns over the use of chloride-containing storage solvents are addressed by several corrosion studies. Comparisons are made to the previously recommended storage/shipping solvent of 20% methanol in water. The five different shipping/storage solvents compared for use with BioResolve SEC mAb, 200 Å, 2.5 µm 4.6 x 150 mm Columns are:
A: 10% acetonitrile/90% 25 mM Na-PO4, pH 7.0 with 100 mM KCl
B: 10% acetonitrile/90% 2.5 mM Na-PO4, pH 7.0 with 10 mM KCl
C: 10% acetonitrile/90% 20 mM Na-PO4, pH 6.8
D: 10% acetonitrile/90% Milli-Q water
E: 20% methanol/80% Milli-Q water
In this study, a total of 15 columns were packed and tested using a 50 mM Na-PO4, pH 7.0, 200 mM KCl mobile phase with the Waters mAb Size Variant Standard (p/n: 186009429). After the initial testing of all 15 columns, sets of three columns were flushed with 10 column volumes (CV) into each of the above five storage solvents. After the initial testing of the 15 columns, one column from each storage solvent group was retested after one, two, and four months of storage at room temperature.
The Waters mAb Size Variant Standard is supplied with a certificate of analysis for each prepared standard lot. It is comprised of the NISTmAb Reference Material (RM) 8671 (a humanized monoclonal antibody) and non-reduced IdeS digested NISTmAb fragments LMWS2 (~100,000 Da) and LMWS3 (~50,000 Da), two mAb fragments with similar molecular weights as the IdeS fragments are endogenous to NIST RM 8671, LMWS1, and LMWS4, respectively. An example chromatogram of the Waters mAb Size Variant Standard and the NIST RM 8671 is shown in Figure 2. More information, see 720006811EN.
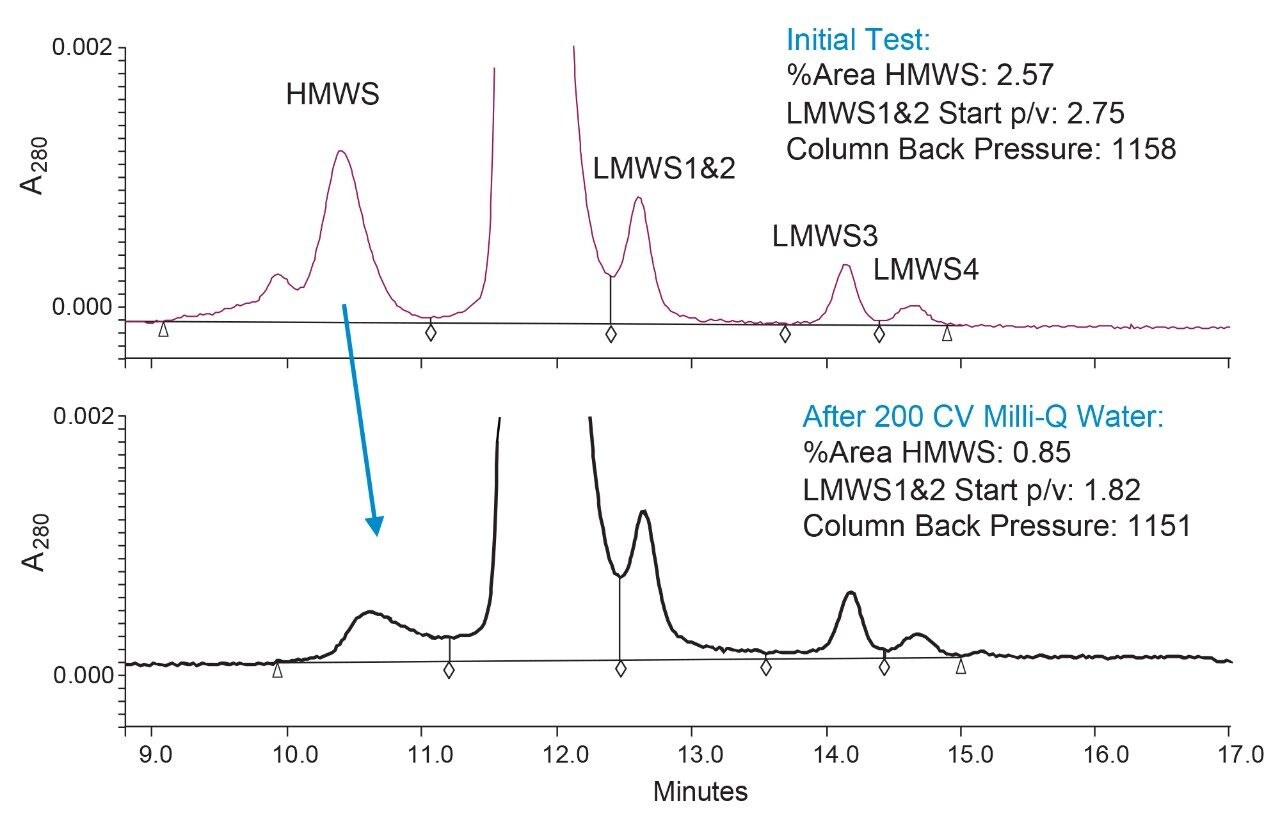
All the investigated storage solvents maintained equivalently unchanged column performance for up to one month. Most of the Waters Empower Chromatography Data System parameters monitored during the four month study did not show significant changes. Minor changes in retention time for the main peak were observed for all solvent groups (<0.2 min). All solvents showed a slight increase in retention which remained steady after two months. These slight changes would have likely to of occurred in a shorter period with routine column use.
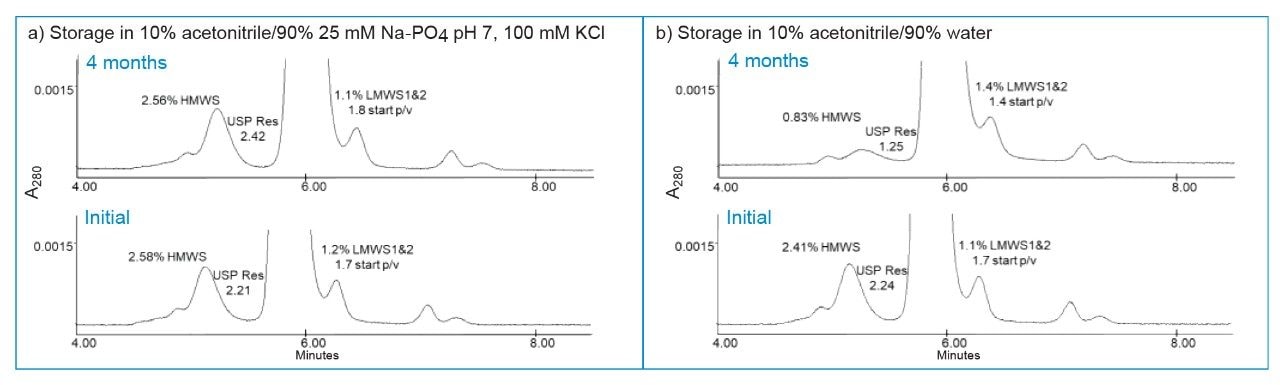
The Empower chromatographic parameters that showed significant changes are shown in Figure 3. The % areas for the HMWS continued to decline (Figure 3a) after one month of storage in solvents that did not contain buffer and/or salt. The dimer-main peak resolution (USP Resolution at Half-Height, USP Res. HH) also continued to decrease over time in these buffer/salt free solvents. Of interest is the contrast illustrated by the use of 10% acetonitrile in water versus the 10% acetonitrile with buffer and salt (Figure 4). The 10% acetonitrile in water showed the largest loss of the HMWS at four months. This is consistent with a previous study (data not shown) that found purging a column with larger amounts (>10 CV) of 20% methanol/80% water showed a similar effect. We did not select methanol as the antimicrobial agent for the BioResolve SEC mAb Column storage solvent due to the impact of unknown low-level impurities in methanol that in the past negatively impacted column performance.
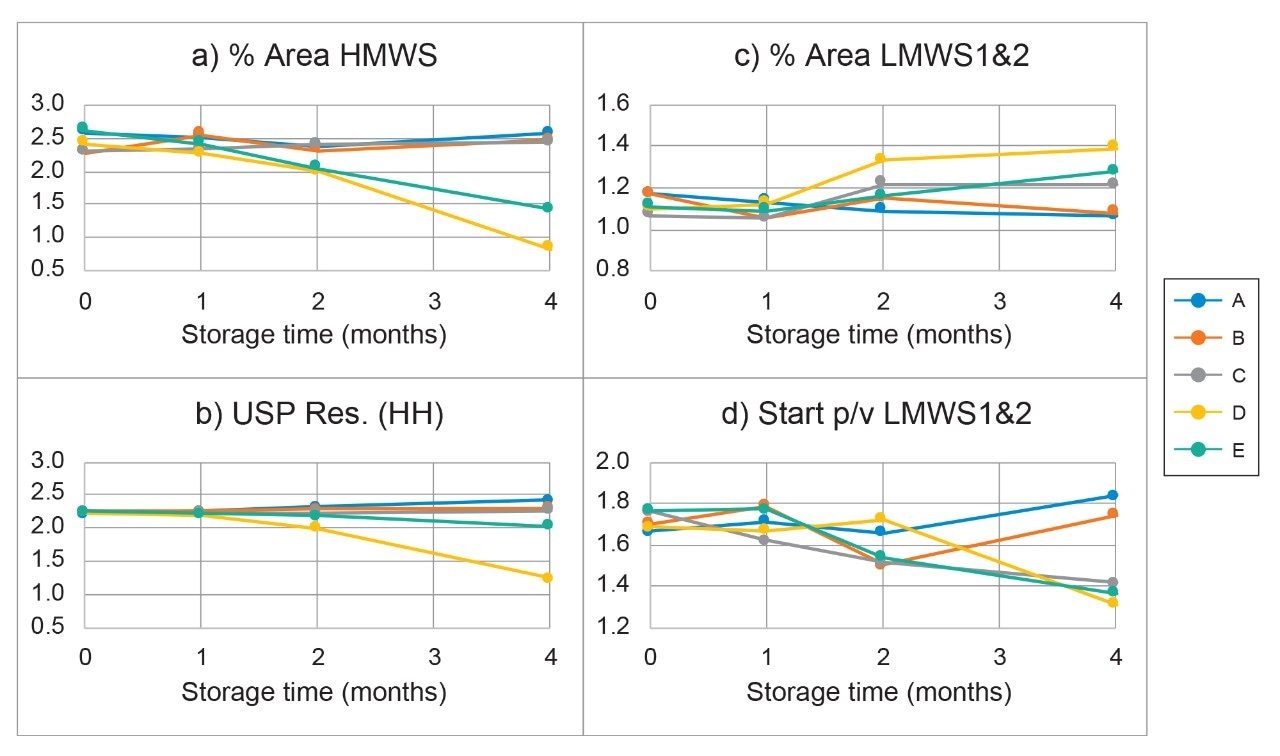
Figures 3c and 3d show the impact of the storage solvent on the % area for LMWS1&2 and its resolution from the main peak. The LMWS1&2 % area increases as a result of decreasing resolution from the main peak. It has been observed that as resolution decreases the % area for LMWS1&2 increases due to the increasing addition of main peak area. This resolution was monitored using the Empower parameter: start peak to valley heights (start p/v) for LMWS1&2 because the more typically used resolution parameter (USP Res. HH) could not be calculated. Again, the storage solvents that did not contain buffer plus salt did not maintain the column’s initial start p/v after a storage period of about two months.
Stainless steel (SS) is a specialized group of steel alloys designed to resist corrosion. All steel components in the fluid path of Waters SEC columns are austenitic 316 SS. This steel is designed to be corrosion resistant. Corrosion of stainless steel in aqueous solutions is a function of pH, halide (chloride) and/or sulfide concentration, and temperature. The corrosion rate of SS is enhanced by lower pHs, increased chloride concentrations, and higher temperatures. Working with the chloride salt containing buffers used in SEC brings up concerns of rusting for SS parts. Rust (iron oxide) forms as an oxidation product of iron. Iron combines with water and oxygen to form the insoluble reddish-brown ferric hydroxide oxide.
We are all familiar with the risk of rust formation in LC systems as well as columns with the use hydrochloric acid (HCl). HCl is frequently used to facilitate aggressive ranking of various types of steel for pitting corrosion. Its aggressive nature is blamed on the combined effects of the acidic environment as well as the chloride ion. The corrosion situation is quite different when the pH is in the range used during SEC (pH 5–8) as is the case for all five of the storage solvents tested. This is thanks to metal oxide/hydroxide layers that are maintained on the metal surface, protecting it from attack. These layers are more readily dissolved under acidic conditions.
Based on extensive experience, the most frequent site for rust formation in columns is the frits. This is due to the higher surface area present compared the column tube wall in addition to the higher oxygen levels at the column inlets and outlets. Other sources of contamination such as microbes, catalysts, or other metals can also accelerate corrosion. In order to check for evidence of frit rusting, all the columns were opened and examined for rust after four months storage in the different storage solvents. None of the storage solutions caused rusting during the four months of storage at ambient temperatures.
Although ASTM G48 is one of the most common standard tests used to rank various metals for corrosion, it relies on conditions (the very acidic ferric chloride solution) that are dissimilar from those required to monitor corrosion under SEC conditions. To assess rusting under our test conditions, frit “soaking” experiments were conducted using:
In this second experiment, 10 mL of each of the above solutions was added to 20 mL scintillation vials. Each solution was set up in duplicate with four, 0.2 µm 316 SS frits. The capped vials were sonicated for 5 min then aged at ambient temperature or 60 °C. The vials were checked under a microscope periodically for seven weeks. During this time period there was no evidence of rust formation even at 60 °C. After the seven weeks, all the vials were maintained at ambient temperature for nine months and still no signs of rust formation were detected.
In the third experiment, ASTM B895 was used. The ASTM B895 test is specifically designed to test porous stainless-steel samples. It consists of soaking porous stainless steel (SS) in 5% NaCl (855 mM) until corrosion occurs. The test was made more aggressive by soaking the frits in an oven at 60 °C. Our SS frits were tested along with other porous materials. After seven weeks submerged in the 5% NaCl the other materials showed a significant amount of rust while our SS frits remained rust-free.
The choice of acetonitrile as the co-solvent in the shipping/storage solvent was based at least in part on its cell toxicity profile as shown by its efficacy in killing an extremophile bacterial strain at 10% acetonitrile.6 Only storage solvents that contained buffer plus salt maintained the initial performance of the BioResolve SEC mAb Columns. The storage solution that performed the best was the 10% acetonitrile/90% 25 mM sodium phosphate pH 7, 100 mM KCl. The more dilute version of this buffer gave very similar performance suggesting that there is range of buffers plus salt that can be useful as storage solvents. It is noteworthy to mention that the column containing only buffer in 10% acetonitrile (no salt) maintained its performance for the HMWS but showed degradation in the main peak/LMWS1&2 resolution. In contrast, the 10% acetonitrile in water showed a 66% loss in HMWS % area, with a 44% decrease in the USP resolution at half height between the dimer and monomer, and a 26% increase in the LMWS1&2 % area that resulted from a 22% decrease in the start p/v resolution. The 20% MeOH/80% water solvent showed similar changes to those of the 10% ACN/90% water but to a lesser degree.
Corrosion testing of the column frits further confirmed the non-corrosive nature of the selected storage solvent. This study suggests that the use of a buffer plus salt environment helps maintain the initial column performance after long-term storage with the use of 10% acetonitrile as the bacteriostatic agent.
The authors would like to thank Steve Shiner and Justin McLaughlin for their roles in the development of BioResolve SEC mAb Columns, and Bill Warren for his guidance and energy in support of this project.
1. Hotchkiss, M. Studies on Salt Action: VI. The Stimulating and Inhibitive Effect of Certain Cations upon Bacterial Growth. J. Bacteriol. 1923, Mar, 8(2), 141–162 [PMC379008].
2. Fabian F. W.; Winslow C. E. The Influence upon Bacterial Viability of Various Anions in Combination with Sodium. J. Bacteriol. 1929 Oct, 18(4), 265–291 [PMC375083].
3. Holm G. E.; Sherman J. M. Salt Effects in Bacterial Growth: I. Preliminary Paper. J. Bacteriol. 1921 Nov, 6(6), 511–519 [PMC378946].
4. Lichstein, H. C.; Soule, M. H. Studies of the Effect of Sodium Azide on Microbic Growth and Respiration I. The Action of Sodium Azide on Microbic Growth. J. Bacteriol. 1944 Mar, 47(3), 221–230 [PMC373903].
5. Maguire E. D.; Wallis, R. B. The Role of Bacterial Contamination in the Isolation of Apparent Anti-Inflammatory Factors from Rabbit Anti Lymphocytic Serum. Br. J. Pharmac. 1977, 59, 261–268 [PMC1667729].
6. Kongpol, A.; Kato, J.; Tajima, T.; Vangnai, A. S. Characterization of Acetonitrile-Tolerant Marine Bacterium Exiguobacterium sp. Microbes Environ. 2012, 27(1), 30–35 [PMC4036024].
7. Xu, G.; Wu, A.; Xiao,L.; Han, R.; Ni, Y. Enhancing Butanol Tolerance of Escherichia coli Reveals Hydrophobic Interaction of Multi‑Tasking Chaperone Sec B. Biotechnol. Biofuels, 2019, 12, 164.
8. Registry of Toxic Effects of Chemical Substances (RTECS), https://www.cdc.gov/niosh-rtecs/VY7AD550.html
9. Bräse, S.; Mende, M.; Jobelius, H. H.; Scharf, H-D. Hydrazoic Acid and Azides, Ullmann's Encyclopedia of Industrial Chemistry, 2015 Wiley‐VCH Verlag GmbH & Co, KGaA.
10. Chang, S.; Lamm, S. H. Human Health Effects of Sodium Azide Exposure: A Literature Review and Analysis. Int. J. Toxicol. 2003 May-Jun , 22(3), 175–86.
11. Current Intelligence Bulletin 13 - Explosive Azide Hazard. Rockville, MD: U.S. Department of Health, Education, and Welfare, Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health, DHEW (NIOSH) Publication No. 78-127, (CIB 13), 1976 Aug, 1–5.
12. Mobile Phase Preparation and Use, Guide to Successful Operation of your LC System, Millipore Corporation 1991. Manual Number 022378 Rev 0, Chapter 4–11.
13. Meyer, V. R. 3rd Ed. Practical High-Performance Liquid Chromatography, 1998, West Sussex, England. John Wiley & Sons Ltd, 75.
14. Link, Jr., G. W.; Keller, P. L. Effects of Solutions Used for Storage of Size-Exclusion Columns on Subsequent Chromatography of Peptides and Proteins. J. Chromatogr., 1985, 331, 253–264.
720007077, November 2020