
In this application note, we describe the targeted analysis of four food allergens in a variety of matrices using Waters ACQUITY UPLC System and Xevo TQ-S. The four allergens investigated in this method were milk (Bos Taurus), egg (Gallus gallus chicken), peanut (Arachis hypogaea) and soybean (Glycine Max (Glycine hispida).
The protocol was tested on processed and complex food matrices including chocolate, ice cream, tomato sauce, and cookies.
Detection levels tested were benchmarked against the levels stated in the AOAC SMPR 2016.002 and VITAL (Voluntary Incidental Trace Allergen labelling) reference doses.
Food allergy is a worldwide health problem affecting both adults and children. To avoid allergic reactions, allergens must be totally excluded from the diet. Consequently, allergic customers can only refer to mandatory labeling to try and avoid coming into contact with the food allergen. However, the undeclared presence of these allergens is still widespread.
To help food industries in the management of hidden allergens, sensitive, specific quantitative, and robust analytical methods need to be developed.
Traditionally techniques such as ELISA and PCR have been used for routine analysis, but in recent years, there has been increasing interest in the utility of LC-MS based methods. In March 2016, AOAC released the first standard method performance requirements (SMPR) specifically for the analysis of four food allergens using LC-MS/MS.1 The detection levels tested are benchmarked against the levels stated in the AOAC SMPR 2016.002 and VITAL (Voluntary Incidental Trace Allergen Labelling)2 reference doses.
In this application note, we describe the targeted analysis of four food allergens in a variety of matrices using Waters ACQUITY UPLC System and Xevo TQ-S.
Figure 1.
1A. MBP-fusion protein of the major peanut allergen Ara h 2: DOI: 10.2210/pdb3ob4/pdb; 1B. Bovine allergen Bos d 2 in the trigonal space group P3221:DOI: 10.2210/pdb4wfu/pdb; 1C. NMR solution structure of soybean allergen Gly m 4: DOI: 10.2210/pdb2k7h/pdb;
1D. Crystal structure of uncleaved ovalbumin at 1.95 angstroms resolution: DOI: 10.2210/pdb1ova/pdb.
Images courtesy of the RSCB Protein Data Bank.
This method is based on a single protocol applicable to the different tested allergens and foodstuffs. Details on the sample preparation step are described elsewhere.3
The four allergens investigated in this method were milk (Bos Taurus), egg (Gallus gallus chicken), peanut (Arachis hypogaea) and soybean (Glycine Max (Glycine hispida).
The protocol was tested on processed and complex food matrices including chocolate, ice cream, tomato sauce, and cookies.
|
LC system: |
ACQUITY UPLC |
|||
|
Column: |
ACQUITY UPLC BEH130, 2.1 x 150 mm |
|||
|
Column temp.: |
40 °C |
|||
|
Sample temp.: |
10 °C |
|||
|
Injection volume: |
20 μL |
|||
|
Flow rate: |
0.2 mL/min |
|||
|
Mobile phase A: |
Water + 0.1% formic acid |
|||
|
Mobile phase B: |
Acetonitrile + 0.1% formic acid |
|||
|
Gradient: |
0 to 1 min: 86% A; 1 to 16.5 min: 86% to 60% A; 16.5 to 16.6 min: 60% to 0% A; 16.6 to 21 min: 0% A; 21.0 to 21.1 min: 0% to 86% A; 21.1 to 24 min: 86% A |
|
MS system: |
Xevo TQ-S |
|||
|
Ionization mode: |
ESI+ in MRM mode |
|||
|
Capillary voltage: |
2.0 kV |
|||
|
Collision gas flow: |
0.12 mL/min |
|||
|
Cone voltage: |
35 V |
|||
|
Cone gas flow: |
150 L/h |
|||
|
Desolvation flow: |
1200 L/h |
|||
|
Source temp.: |
150 °C |
|||
|
Desolvation temp.: |
500 °C |
|||
|
Data solutions: |
Skyline (MacCoss Lab), UniProt, MassLynx |
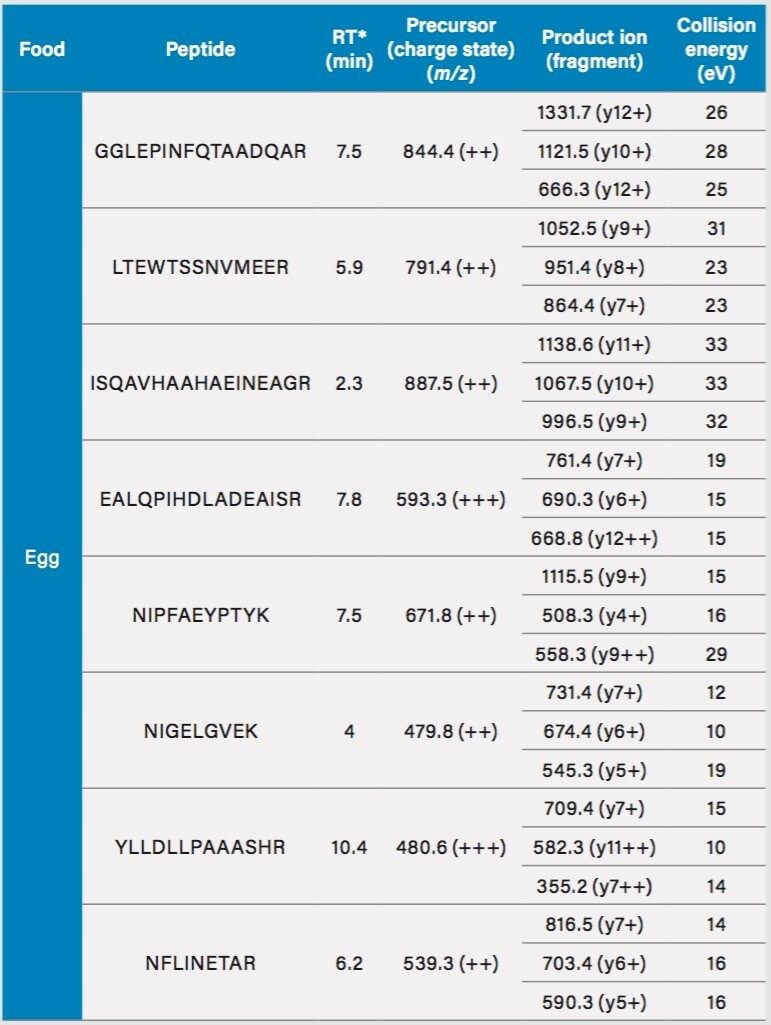
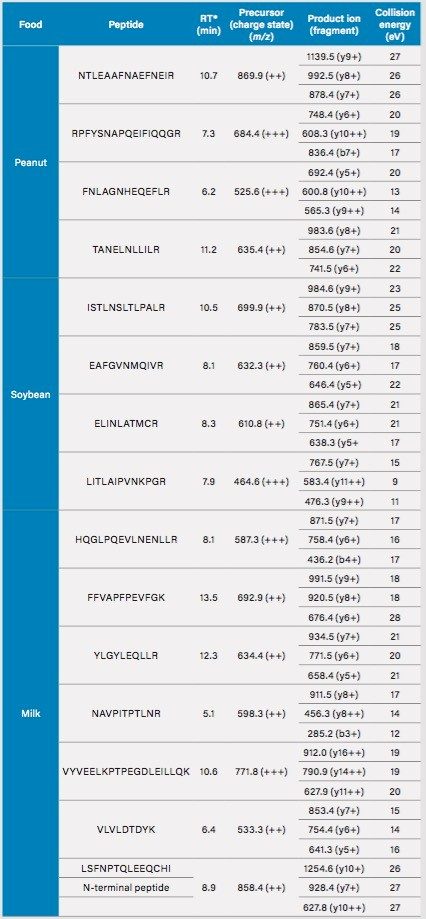
Multiple reaction monitoring (MRM) parameters for the identification of milk, egg, soybean, and peanut proteins by ACQUTIY UPLC and Xevo TQ-S.
*Retention time (RT) is in sauce.
The software package Skyline, was used for in silico enzymatic digestion of food allergen proteins and to help produce potential MRMs for the experiment. From the list produced by Skyline, each MRM was analyzed using the ACQUITY UPLC System coupled to the Xevo TQ-S for sensitivity and reproducibility (in different food matrices). In this method a total of 23 peptides and 69 MRMs were included as part of the analysis, although no regulations as yet state what determines a positive identification of an allergenic protein. (e.g. number of proteins and peptides to be monitored). For egg and milk, peptides representative of the different components of the egg: egg white (ovalbumin) and the yolk (vitellogenin), milk (casein), and whey (β-lactoglobulin), are included in the method.
Current regulations address the analytical levels of detection for gluten, and so for the allergens monitored in this method, levels were assessed from the recommendation levels provided by VITAL and the AOAC SMPR for food allergens.
For each allergen, a single, common LOQ was determined for all targeted matrices (Figure 2). For each peptide, two MRM transitions in allergen-free matrices and incurred matrices were shown to demonstrate the specificity of the method and to confirm detection of the food allergens at the LOQ. The LOQ was defined as the minimum concentration giving a signal-to-noise ratio (S/N) of 10 for the most intense MRM transition of the targeted food allergen. The sensitivity of detection for the food allergen peptides was determined on the worst case, mainly processed cookies. The LOQs recorded are: 0.5 mg milk proteins/kg for caseins, 5 mg milk proteins/kg for whey, 3.4 mg egg proteins/kg for egg white, 30.8 mg egg proteins/kg for egg yolk, 2.5 mg/kg for peanut proteins, and 5 mg/kg for soybean proteins.
Linearity and matrix effects were tested by analyzing three independent foodstuff preparations (incurred chocolate and ice cream and processed cookies and sauce) that contained different concentrations of milk, egg, soy, and peanut food allergen proteins (Figure 3).
Although the matrix effect and the effect of the thermal process were not the same for both targeted peptides from the same food allergen, the linear coefficient of regression supported the reliability of the method even the absence of an internal standard.
Sensitive detection of food allergens (milk casein, whey, egg white, egg yolk, peanut, and soybean) was achieved by analyzing food allergen peptides using the ACQUITY UPLC System coupled to the Xevo TQ-S.
In keeping with food production requirements, the targeted matrices were processed (tomato sauce, cookies) or incurred (chocolate, ice cream). This multi-allergen detection method has the lowest limits of quantification available to date (expressed in total proteins and not soluble proteins): 0.5 mg milk proteins/kg for caseins, 5 mg milk proteins/kg for whey, 3.4 mg egg proteins/kg for egg white, 30.8 mg egg proteins/kg for egg yolk, 2.5 mg peanut proteins/kg, and 5 mg soybean proteins/kg.
While matrix effects can be observed from the data shown, further work will involve the inclusion of internal standards in order to make the method quantitative.
720005892, February 2017