For forensic toxicology use only.
This is an Application Brief and does not contain a detailed Experimental section.
This application brief assess the feasibility of using collision cross section (CCS) data to facilitate differentiation of two isomeric synthetic cannabinoids.
Collision cross section differentiates the isomeric synthetic cannabinoids, JWH-015 and JWH-073.
In recent years there has been a steady increase in the use of synthetic cannabinoids for recreational purposes. These substances are designed to mimic the effects of delta-9 tetrahydrocannabinol (delta-9-THC)—the main psychoactive element of marijuana, however these analogs can be significantly more potent. Since the first synthetic cannabinoid was detected in a “legal-high” product in 2008, this group of substances has continued to expand year on year; and currently more than 160 synthetic cannabinoids are being monitored by the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) through the European Union’s early warning system.¹ The rapid evolution of this class of substances, as well as their chemical diversity, poses a challenge for the analytical laboratory. Consequently, scientists are increasingly looking to techniques based on high-resolution mass spectrometry to provide unambiguous compound identification.
Structurally, the synthetic cannabinoids can generally be broken down into four key parts: the core and substituents; the link section; the ring and substituents; and the tail section. Although several substances share the same elemental formula, the overall structural arrangement of the molecule can be different. While screening techniques based on Tof-MSE provide accurate mass of the precursor species as well as fragment ions, ion mobility mass spectrometry (IMS) offers the potential for an extra dimension of separation based on the movement of ions through a neutral gas.2,3 Movement is dependent on the ion’s size, shape, and charge. Thus, an ion’s drift time and subsequently-derived collision cross section (CCS; Ω) may increase specificity of identification. In this study, we assessed the feasibility of using CCS to facilitate the differentiation of two isomeric cannabinoids.

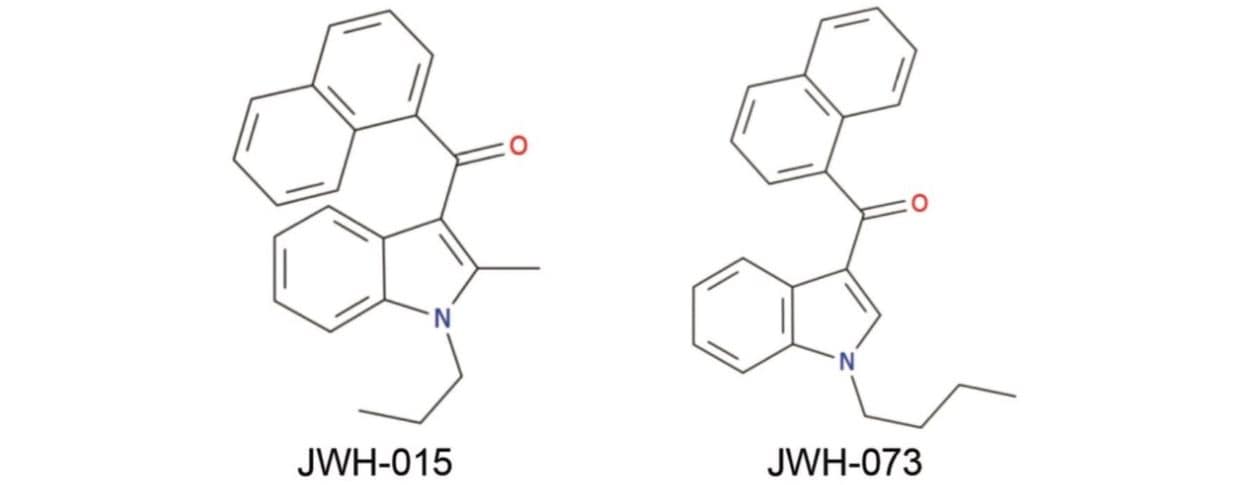
Synthetic cannabinoids JWH-015 and -073 were obtained from Sigma-Aldrich (Poole, UK) as individual solutions at a concentration of 1 mg/mL. Working solutions were prepared by dilution with mobile phase A.
Chromatography was achieved using the Waters ACQUITY UPLC I-Class System and a previously described chromatographic method.² Mass spectrometry analysis was achieved using the Waters Vion IMS QTof, operated in high definition MSE (HDMSE) which enabled collection of IMS data as well as accurate mass data.
Individual dilutions of the synthetic cannabinoids JWH-015 and -073 (100 ng/mL), were analyzed in replicate (n=4) to establish reference CCS. This information was added into an existing scientific library to complement other identification parameters (Table 1).
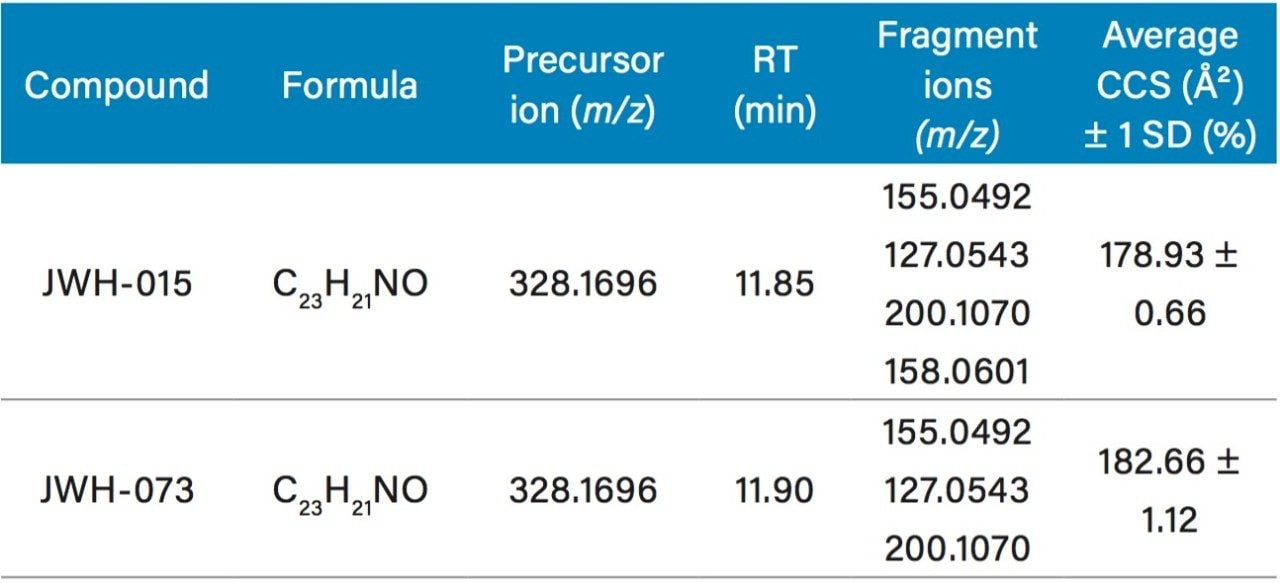
As shown in the table, these two substances are isobaric, having identical masses for both the precursor and several high-energy fragment ions. Furthermore, under the applied chromatographic conditions they elute very closely – thus, unequivocal identification could be challenging even with high-resolution mass spectrometry.
However, the measured CCS values were different and highly reproducible. To determine if the application of CCS could provide a simple means to differentiate the two substances, a mixture of JWH-015 and JWH-073 was analyzed.
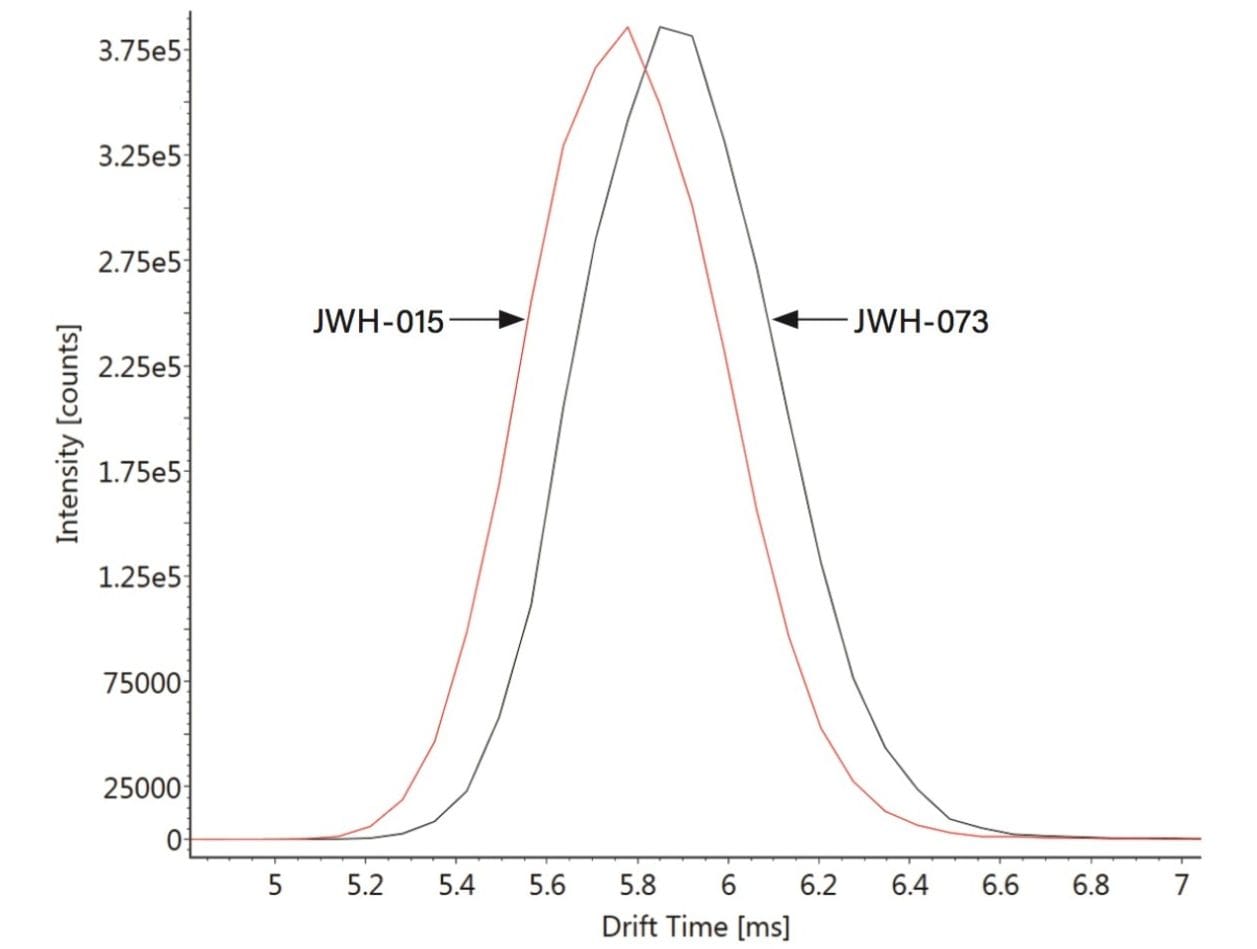
Figure 2 shows the mobilograms obtained, and confirms that the movement of these two analogues through the IMS region of the instrument is different. The most likely explanation is owing to the small difference in conformation – e.g., JWH-073 has a slightly longer alkyl tail than JWH-015. Because the latter is more compact it is able to move through the IMS region faster.
The data was processed using Waters UNIFI Scientific Information System, which automatically compares acquired data with the scientific library. Identification is based on a combination of retention time (typically within 0.35 minutes of the reference) and accurate mass of precursor and fragment ions (typically within 5 ppm of expected). However, for IMS QTof data, results can be further filtered by applying the CCS reference ± 1% tolerance. Application of this parameter led to the unambiguous identification of both substances within the mixture.
Collision cross section is a valuable parameter in compound identification. The example illustrated in this note shows the benefit of using CCS to distinguish between isomeric substances and increase specificity. The use of drift time alignment facilitates the removal of interfering ions from both the low and high energy spectra, providing cleaner spectra and consequently reducing the occurrence of false positive identifications.
720005827, February 2017