This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates the high reproducibility of ProteinWorks eXpress Digest Kits resulting from a standardized protocol and pre-weighed, lot-traceable reagents.
Protein quantification workflows are not only time consuming and complex, but due to their elaborate, multi-segment nature, the margin for error and the potential variability across sites, analysts, and even day-to-day can be very high. Poor reproducibility in protein quantification analytical data and a general lack of expertise strongly support the requirement for a standardized, kit-based approach.
In any bioanalytical assay, one of the greatest sources of variability arises from the sample preparation. This is especially true for protein quantification workflows which often contain many segments-each with multiple steps capable of introducing variability. This can be of particular concern when assays are transferred from sponsor-to-CRO or from lab-to-lab within the same company, or across sites for example. Furthermore, given the multitude of possible options within a typical workflow, method development time and the expertise required are significant. The difficulty in implementing these assays is further aggravated by the complexity of the troubleshooting required when analytical goals are not met. At the same time, an assay is expected to meet acceptable accuracy and precision guidelines and provide reliable, reproducible results to make critical research and discovery-stage decisions. Thus, there is a strong need for simpler, more standardized workflows. These would ideally employ generic, kitted methods that provide a “recipe” and the reagents necessary to streamline the workflow, reduce variability and allow for implementation by less experienced scientists.
ProteinWorks eXpress Digest Kits are flexible, broadly applicable, sample preparation kits containing pre-measured, lot traceable reagents optimized for the accurate, precise, reproducible and robust LC-MS quantification of proteins via the surrogate peptide approach.
Reproducibility within an assay (intra-kit) and between assays (inter-kit) was evaluated with two (2) analysts, on different days, using a total of five (5) unique lots of kits, and six (6) technical replicates per kit. Both the ProteinWorks eXpress Direct Digest Kit (p/n 176003688), for direct digestion of whole plasma, and ProteinWorks eXpress Digest Kit (p/n 176003689), for digestion of affinity-purified plasma, were employed. Using the included generic protocol, several signature peptides (a combination of both generic and unique), resulting from the digestion of the monoclonal antibody drug bevacizumab (Avastin) in plasma, were evaluated and analyzed by LC-MS. The coefficient of variation (CV), also known as relative standard deviation (RSD), was used to evaluate reproducibility, as high CV values are indicative of poor reproducibility and precision. Raw area counts for multiple tryptic peptides from the aforementioned protein were used to make the assessment.
For the two types of kits, both intra-kit and interkit % CV’s on average were ≤10. Figures 1A and 2A summarize intra and inter-kit reproducibility data from direct digestion of whole plasma using ProteinWorks eXpress Direct Digest Kits. Figures 1B and 2B summarize intra and inter-kit reproducibility data from affinity purified plasma using ProteinWorks eXpress Digest Kits. Additionally, using two (2) analysts, on two (2) separate days, the calculated mAb concentrations of the multiple tryptic peptides, when compared, were within 10% of each other, with % CV’s across the six (6)technical replicates ≤15. Data from direct plasma digests are shown in Figure 3A, while data from affinity purified plasma digests are shown in Figure 3B.
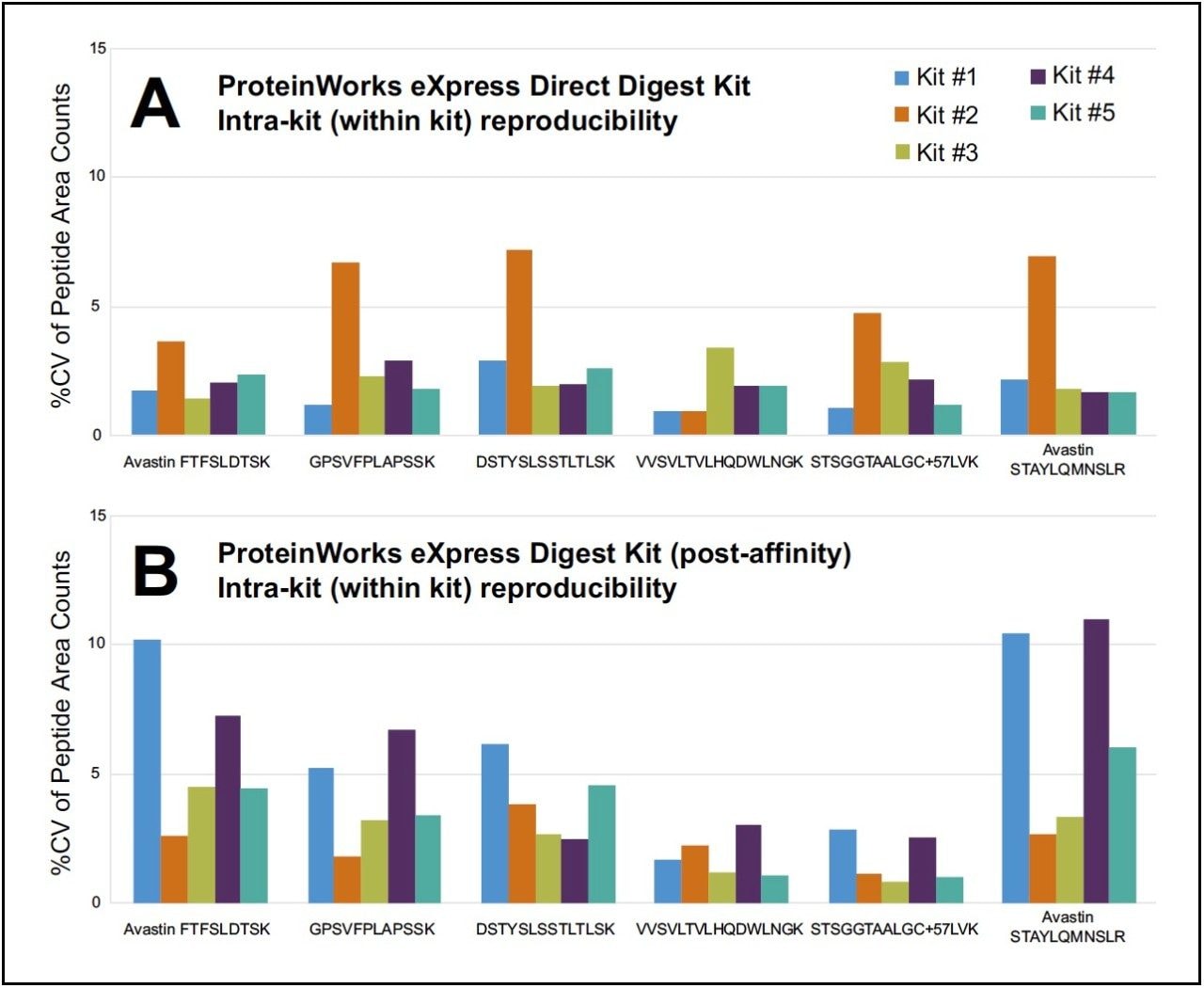
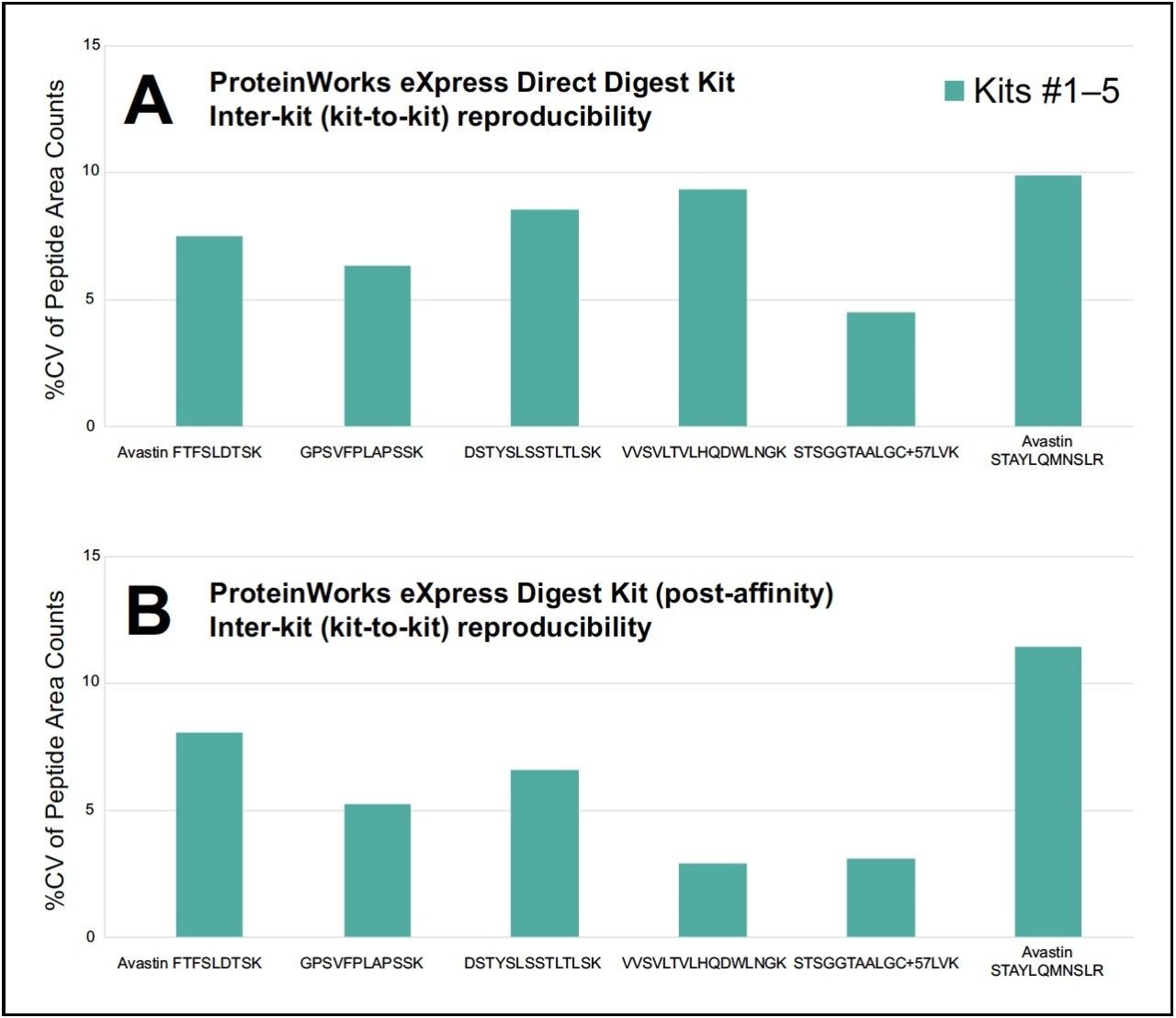
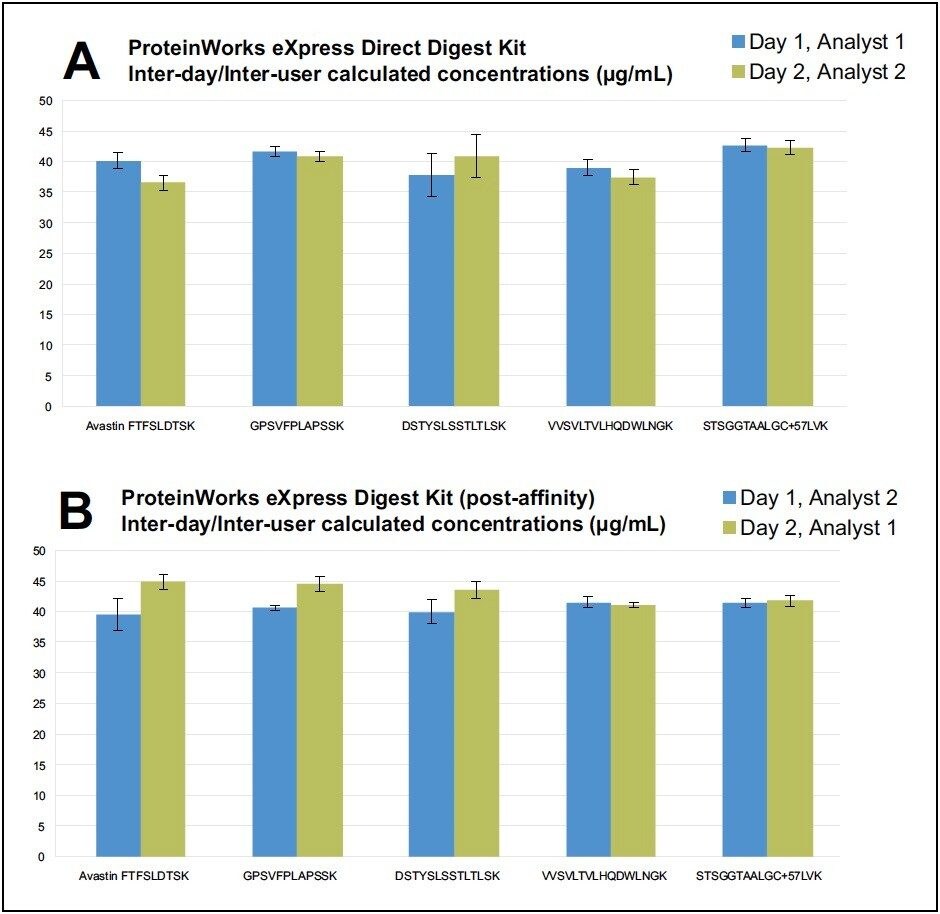
This work demonstrates that single digit reproducibility, accuracy and precision is achievable using this kit-based approach to protein quantification. These data suggest that a high degree of standardization can be achieved across analysts and sites implementing the kit strategy outlined herein.
720005570, January 2016