
In this application note, a rapid and efficient method for the extraction of PAHs from a complex avian egg matrix was developed using established QuEChERS methodology and a one-step protein and phospholipid clean-up using Waters Ostro 96-well plates. This novel sample preparation approach eliminates the use of conventional GPC followed by SPE cleanup and successfully reduces sample preparation time to hours, instead of several days.
The British Petroleum offshore rig explosion on April 20, 2010 released an estimated 4.9 billion barrels of crude oil into the Gulf of Mexico, raising immediate environmental concerns regarding potential threats to the inhabiting wildlife and surrounding ecosystem.1 Polycyclic Aromatic Hydrocarbons (PAHs) are a large group of organic compounds present in crude oil, consisting of two or more aromatic rings fused together.2 PAHs are highly toxic, and while metabolizable they have been shown to bio-accumulate, especially heavier molecular weight PAHs. PAHs are highly carcinogenic and mutagenic compounds that have been shown to generate reactive oxygen species.3 Owing to their lipophilic nature, PAHs readily accumulate in eggs where residues may be orders of magnitude higher than in other organs, including the liver.4 While sufficient data are available regarding the carcinogenicity of the PAHs, other long-term toxicological effects, including neurotoxicity, are not as well documented.5 Therefore, the development of new, rapid, and sensitive techniques for analysis of PAHs in various tissues would therefore be beneficial.
Commonly used sample preparation techniques for the isolation of PAHs from various matrices are liquid-liquid extraction, basic digestion in KOH followed by gel permeation chromatography and solid-phase extraction (SPE).3 Recently, a more modern high-throughput QuEChERS method was used by our research group for the determination of PAHs in avian eggs and blood.1 A similar approach was used to screen for PAHs in seafood.6 The simplicity of the approach, its effectiveness, and its speed provided an excellent alternative to traditional, lengthy extraction methods. However, the complexity of the biological matrix with high protein and phospholipid content as well as low concentrations of PAHs, meant further extensive cleanup and enrichment was required prior to analysis. This was performed using gel permeation chromatography (GPC) followed by additional SPE to minimize matrix effects which extended the total sample preparation time to several days.7
A novel, one-step protein and phospholipid clean-up using Waters Ostro Pass-through 96-well Plates served as an alternative to a GPC and SPE approaches. It has been shown that sample preparation using Ostro 96-well plates can provide an effective means for the removal of phospholipids in plasma and serum allowing for more sensitive analyses, increased sample throughput, and reduced instrument downtime.8 Our research group utilized a similar approach with Ostro 96-well Plate Technology, coupled with QuEChERS dispersive extraction, to develop and validate a rapid, high-throughput method for the preparation of eggs for the analysis of PAHs.
The most widely used analytical techniques for the quantification of PAHs are HPLC with fluorescence detection and GC-MS. Other analytical approaches have been employed including APPI-LC-MS/MS and GC-MS/MS.6 The work described here shows that the desired chromatographic resolution and detection limits were achieved using a Waters ACQUITY UPLC System equipped with PDA detection.
|
LC system: |
ACQUITY UPLC |
|
Column: |
ACQUITY UPLC CSH C18 2.1 X 100 mm, 1.7 μm |
|
Column temp.: |
50 °C |
|
Sample temp.: |
20 °C |
|
Mobile phase A: |
80:20:00 |
|
Water: |
Methanol |
|
Mobile phase B: |
90:10:00 |
|
Acetonitrile: |
Methanol |
|
Total runtime: |
16 min |
|
Injection volume: |
8.5 μL |
|
Time (min) |
Flow (mL/min) |
%A |
%B |
|---|---|---|---|
|
0 |
0.6 |
85 |
15 |
|
0.25 |
0.6 |
66 |
34 |
|
4 |
0.65 |
61 |
39 |
|
13 |
0.65 |
35 |
65 |
|
13.5 |
0.65 |
3 |
97 |
|
15 |
0.6 |
85 |
15 |
|
UV detector: |
ACQUITY UPLC PDA |
|
Mode: |
Scan/3D mode |
|
Sampling rate: |
20 pts/s |
|
Resolution: |
1.2 nm |
|
Range: |
200 to 450 nm |
In this application note, a QuEChERS extraction method in conjunction with the novel Ostro Pass-through 96-well Plate clean-up was developed for rapid analysis of PAHs in avian egg samples. The use of this methodology greatly reduced sample preparation time from a three-day process to just three hours by avoiding the need to use GPC and SPE.
Certified PAH standards – QTM PAH mix (total of 16 PAHs – cat. # 47930-U) with concentrations of 2.0 mg/mL and EPA 525 mix A (total of 12 PAHs – cat. # 48953-U) with concentration of 500 µg/mL – were both obtained from Supelco and served as the initial stock solutions. The surrogates (perylene-d12 and naphthalene-d8), as well as the internal standard (chrysene-d12), were purchased as neat materials and initial stock solutions of 1.0 mg/mL each were prepared in acetonitrile. All working standards were prepared from stock solutions by serial dilution in acetonitrile and stored in amber vials at 4 °C.
Due to unavailability of a commercially certified source, chicken eggs were obtained from a local farm and these served as the matrix for the preparation of the standard reference material (SRM). The avian egg samples for analysis were collected by our collaborators; the U.S. Geological Survey, U.S. Fish and Wildlife Service, and Minnesota Department of Natural Resources.
Weigh approximately 1.0 g of homogenized egg sample into an 8-mL disposable glass vial. Spike the samples with surrogate compounds and QC standards as needed. Add 5 mL of 1% formic acid in acetonitrile to all samples. In order to ensure an effective interaction between the sample and the solvent, vortex the vials to disrupt the formed egg mass. Add 1.5 g of QuEChERS powder (0.3 g sodium acetate + 1.2 g magnesium sulfate) to all samples and shake vigorously for 1 to 2 min. Centrifuge the vials at 3000 RPMs for 3 minutes.
Take 0.5 mL of the top organic layer and transfer it into an Ostro Pass-through 96-well Plate for cleanup. Spike the sample with the internal standard solution and apply 10 to 15 psi pressure to draw the sample through the Ostro Pass-through Plate. Collect the eluate. To ensure complete transfer of PAHs through the Ostro material, pass an additional 0.25 mL of 100% acetonitrile through the well and collect eluate. Combine both eluates and transfer into LC vials or inject directly from the collection plate.
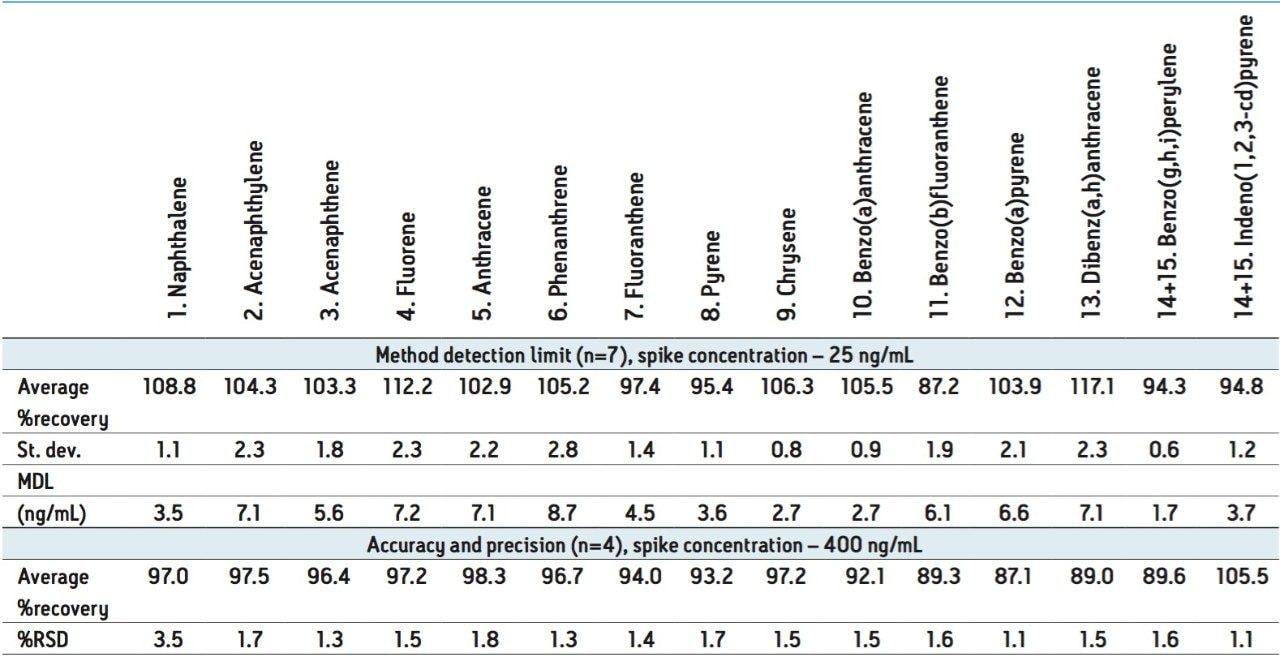
Data was acquired using an ACQUITY PDA Detector operating (scanning 200 to 450 nm) under MassLynx Software v.4.1 control, followed by processing with QuanLynx. The λmax for each of the compounds are listed in Table 1. The correlation coefficient, R2, ranged from 0.9972 to 0.9999 for all the analytes over three orders of magnitude. The method detection limit (MDL), accuracy and precision were calculated using results from the analysis of fortified chicken egg extracts injected in replicate. Standard Environmental Protection Agency protocols were followed and the results are shown in Table 2.

Table 1. The retention times, λmax and R2 value for EPA’s 16 priority pollutant PAHs, surrogate (SUR.) and internal standard (IS) compounds.
*2-bromonaphthalene and benzo(k)fluoranthene are part of the QTM PAH mixture, but were not a part of the analytical procedure, so consequently were omitted from the table. QTM PAH mix includes all PAHs; EPA 525 mix A is missing compounds marked with an asterisk.
As part of the initial evaluation step, a more generic technique was tested with the egg sample and acetonitrile being directly mixed inside the Ostro Pass-through 96-well Plate. This approach was unsuccessful since the viscous mixture did not allow the extraction solvent to pass through the plate. Additionally, aspiration and dispensing of such a viscous matrix was troublesome and a more effective method had to be employed. It was apparent that a completely new approach was required to utilize the Ostro Pass-through 96-well Plate for this application. The combination of a quick and reliable QuEChERS extraction as the first step, followed by the Ostro cleanup, provided the desirable results. Acidified acetonitrile solution was initially added to the sample in order to “crash” out the proteins and served as a preliminary clean-up step. QuEChERS powder (magnesium sulfate and sodium acetate mix) was added to absorb the water present in the sample. To ensure the accuracy of results, quality control standards were added to samples prior to the addition of QuEChERS salts. Any analyte losses could therefore be accounted for from the earliest point in the sample preparation process.
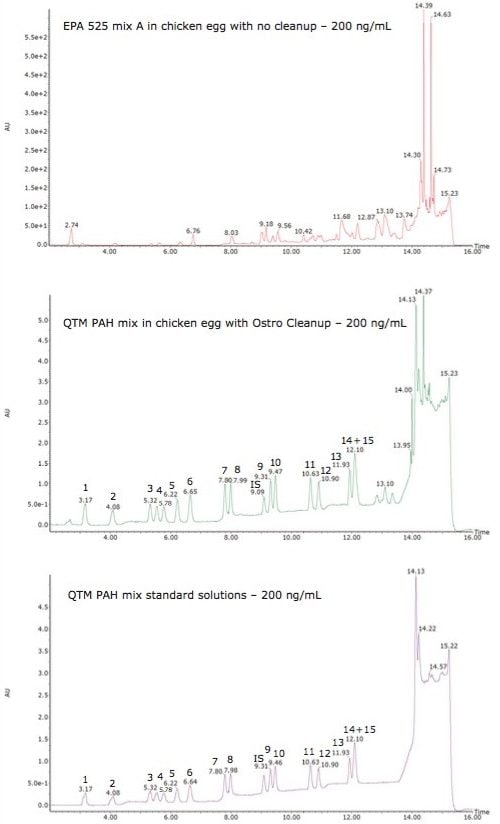
The extraction efficiency of this new method is presented in the Table 2. It needs to be emphasized that a PDA detector was utilized for the quantification of PAHs which provided not only acceptable low detection limits associated with this analytical technique, but also high accuracy and precision. The recoveries for the MDL study were in the range of 87.2 to 117.1%, providing detection limits between 1.7 to 8.7 ng/mL. These low limits were achieved without incorporating a pre-concentration step prior to analysis as commonly used in most traditional techniques. In fact, samples are effectively diluted during this sample preparation protocol and this is only possible because of the extensive clean-up offered by the Ostro material. Figure 2 demonstrates the efficacy of this sample preparation protocol by comparing chromatograms resulting from chicken egg extracts with and without cleanup using Ostro material.
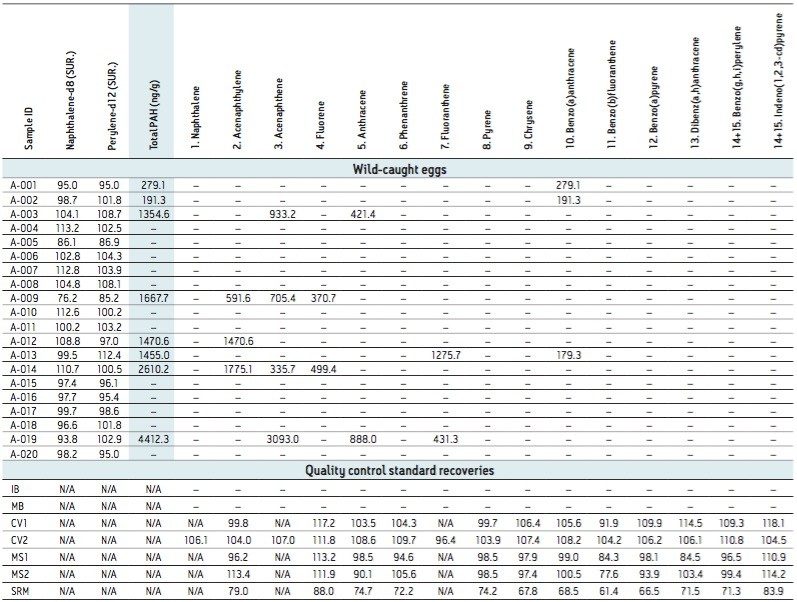
A set of 20 wild-caught eggs were analyzed as part of this development study and the results are shown in Table 3. For the matrix spike samples (MS1 and MS2) spiked with 200 ng/mL concentration of EPA 525 mix A, the method resulted in recoveries for all analytes ranging between 84.3 to 113.4%. Calibration verification standards CV1 (QTM PAH mix) and CV2 (EPA 525 mix A), at a concentration of 200 ng/mL, confirmed the linearity of the calibration curve and provided a check for any analyte degradation during sample preparation. With the recoveries ranging between 91.9% and 117.2%, no adjustment was necessary to account for degradation in these analyses. The analyte recoveries in the set of wild-caught egg samples were reported as total PAHs in ng/g (by wet weight). Individual compounds quantified at greater than the MDL are shown in Table 3.
The combination of QuEChERS extraction and Ostro Pass-through clean-up technologies proved to be an effective, efficient, and sensitive technique for analysis of 16 priority PAHs in an extremely complex biological matrix. This method provided excellent recoveries from the fortified wild-caught egg samples while minimizing matrix effects. In comparison to traditional extraction methods which use GPC followed by SPE cleanup and take several days to complete, this validated method reduced sample preparation time to just three hours for a batch of 20 samples. The reduced preparation time and high-throughput of the method resulted in increased laboratory productivity and a significant reduction in sample preparation costs. This method also required significantly less solvent volume which resulted in a more environmentally-friendly process. The simplicity of our developed and validated method, its robustness, and reproducibility, make it a viable alternative to more traditional approaches such as GPC and other normal phase cleanup strategies.
720004779, November 2014