
This is an Application Brief and does not contain a detailed Experimental section.
This application highlight demonstrates an assay for the analysis of abacavir and related substances that was successfully transferred from an Agilent 1100 Series LC System to an ACQUITY UPLC H-Class System.
When transferring a method to a different HPLC/UHPLC instrument, the new instrumentation must provide the same separation and meet all of the system suitability requirements of the original method/instrument. For gradient separations, the impact of dwell volume can be dramatic and should be considered when transferring a method across chromatographic instruments.
|
Sample: |
Abacavir and related substances |
|
Sample diluent: |
Water |
|
Systems: |
1. Agilent 1100 Series LC System 2. ACQUITY UPLC H-Class System with ACQUITY UPLC PDA Detector and CH-A |
|
Transfer: |
Gradient delay was entered using SmartStart Technology |
|
Wavelength: |
245 nm |
|
Column temp.: |
36 °C |
|
Column: |
CORTECS C18, 2.7 μm, 4.6 x 75 mm |
|
Mobile phase A: |
0.1% Trifluoroacetic acid in water |
|
Mobile phase B: |
85% Methanol in water |
|
Flow rate: |
1.85 mL/min |
|
Injection volume: |
5 μL |
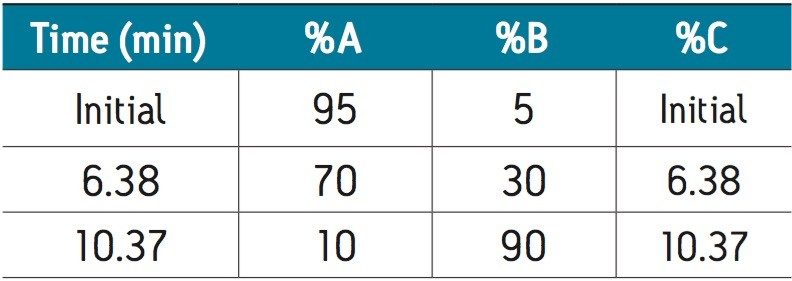
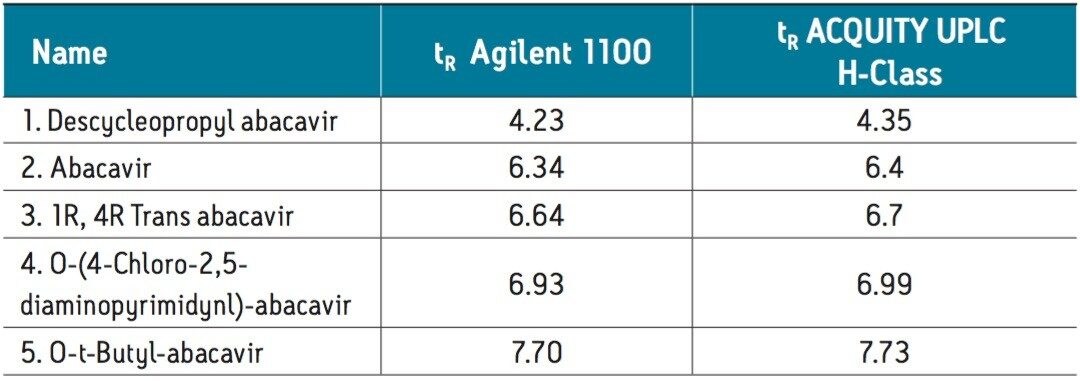
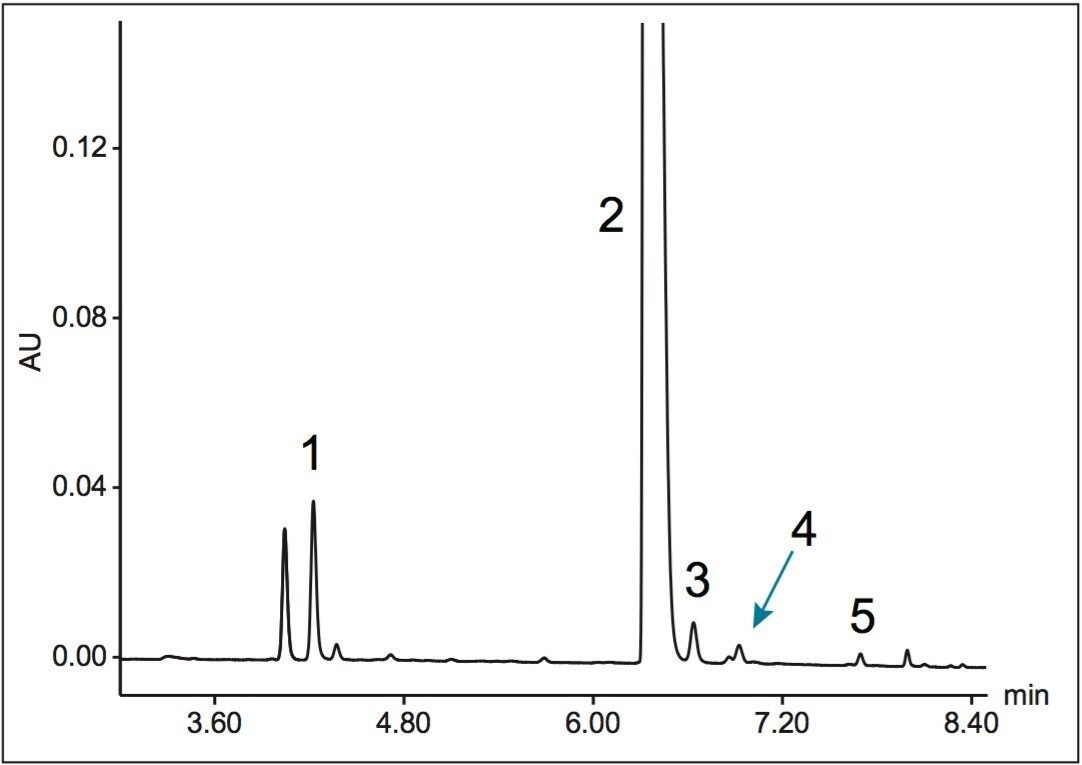
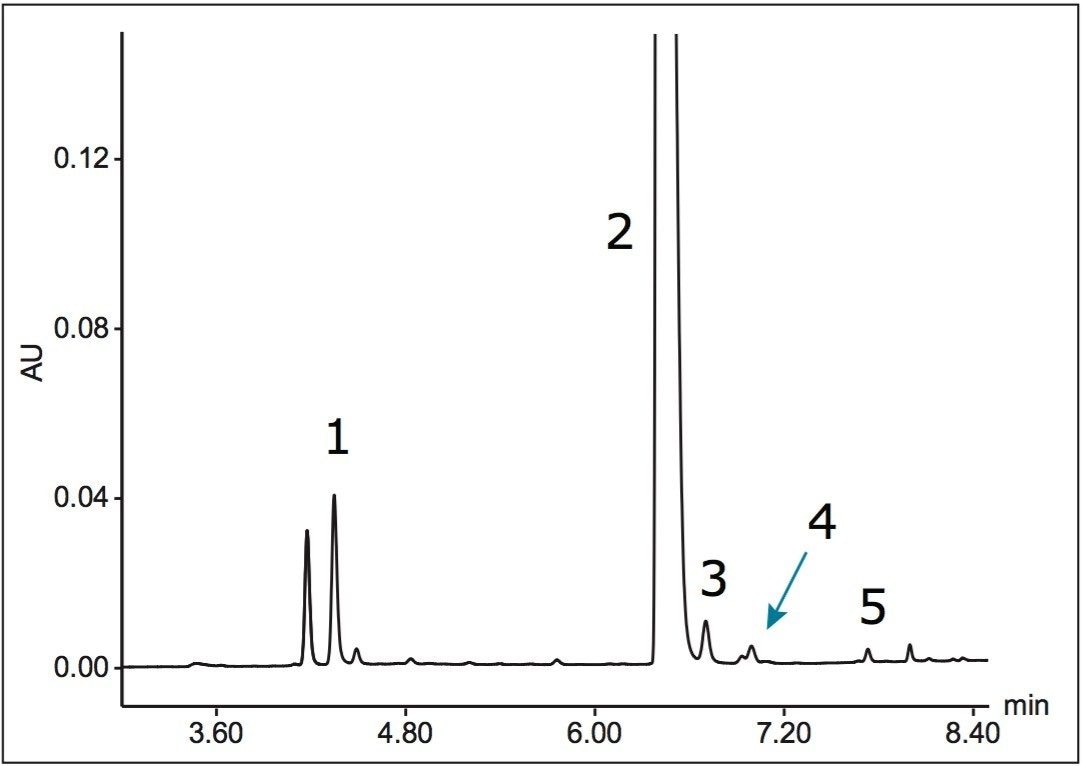
An assay for the analysis of abacavir and related substances was successfully transferred from an Agilent 1100 Series LC System to an ACQUITY UPLC H-Class System. Using gradient SmartStart Technology in the instrument method, the differences in system volume were factored into the method. No changes to the gradient table were required.
1. For complete experimental details, refer to full technical brief 720005252EN at waters.com
720005253, December 2014