This application note demonstrates the use of convergence chromatography in the development and validation of a highly sensitive UPC2-MS/MS assay for the direct analysis of clopidogrel in human plasma from a hexane LLE preparation.
Direct injection of bioanalytical liquid-liquid extracts (LLE) onto UPC2-MS instrumentation without the need for evaporation and reconstitution.
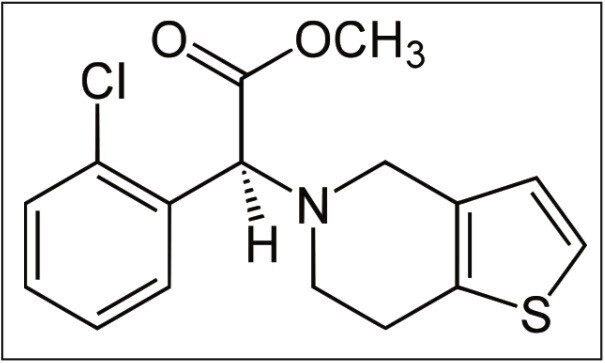
Clopidogrel, shown in Figure 1, is a thienopyridine derivative antiplatelet pro-drug used in the prevention of artherosclerotic events. Following oral administration, the dosed compound undergoes hepatic metabolism to give rise to the active thiol-metabolite and the inactive carboxylic acid metabolite. The inactive metabolite accounts for the majority of circulating clopidogrel-related material in humans, while the active metabolite and unchanged pro-drug are present at very low levels. The mechanism of action is derived from the binding of the active thiol metabolite to cell receptor P2Y12, irreversibly inhibiting the platelet activation process.1
Many bioanalytical methods utilize liquid-liquid extraction (LLE) by incorporating a non-polar solvent, such as hexane or methyl-tert-butyl ether. The widespread utilization of this choice of sample preparation is due to the ability of LLE to produce a much cleaner extract compared to protein precipitation techniques. In addition, LLE methods are relatively inexpensive when compared to other sample preparation methods.2 However, the use of LLE requires dry-down and reconstitution steps into a more polar solvent that is readily compatible with typical starting conditions of revered-phase LC, which is the most common form of LC utilized in bioanalytical LC-MS/MS analysis.
This application note demonstrates the use of convergence chromatography in the development and validation of a highly sensitive UPC2-MS/MS assay for the direct analysis of clopidogrel in human plasma from a hexane LLE preparation.
200 μL of human plasma was mixed with 20 μL of internal standard, then extracted with 600 μL of hexane. Samples were vortex mixed, centrifuged, and then the supernatant was transferred to a total recovery LC vial.
A 10-μL sample was injected onto an ACQUITY UPC2 System. Chromatography was performed on an ACQUITY UPC2 BEH 3.0 x 100 mm, 1.7 μm Column maintained at 40 °C. The column was operated under linear gradient conditions, starting at 98:2 CO2 /0.1% formic acid in acetonitrile to 70:30 in 2 min at a flow rate of 1.4 mL/min.
The column effluent was monitored using a Xevo TQ-S Mass Spectrometer operated in multiple reaction monitoring (MRM) positive ion electrospray mode. The transition 322 ⟶ 212 was employed for the clopidogrel and the transition 326 ⟶ 216 was employed for the d4 internal standard.
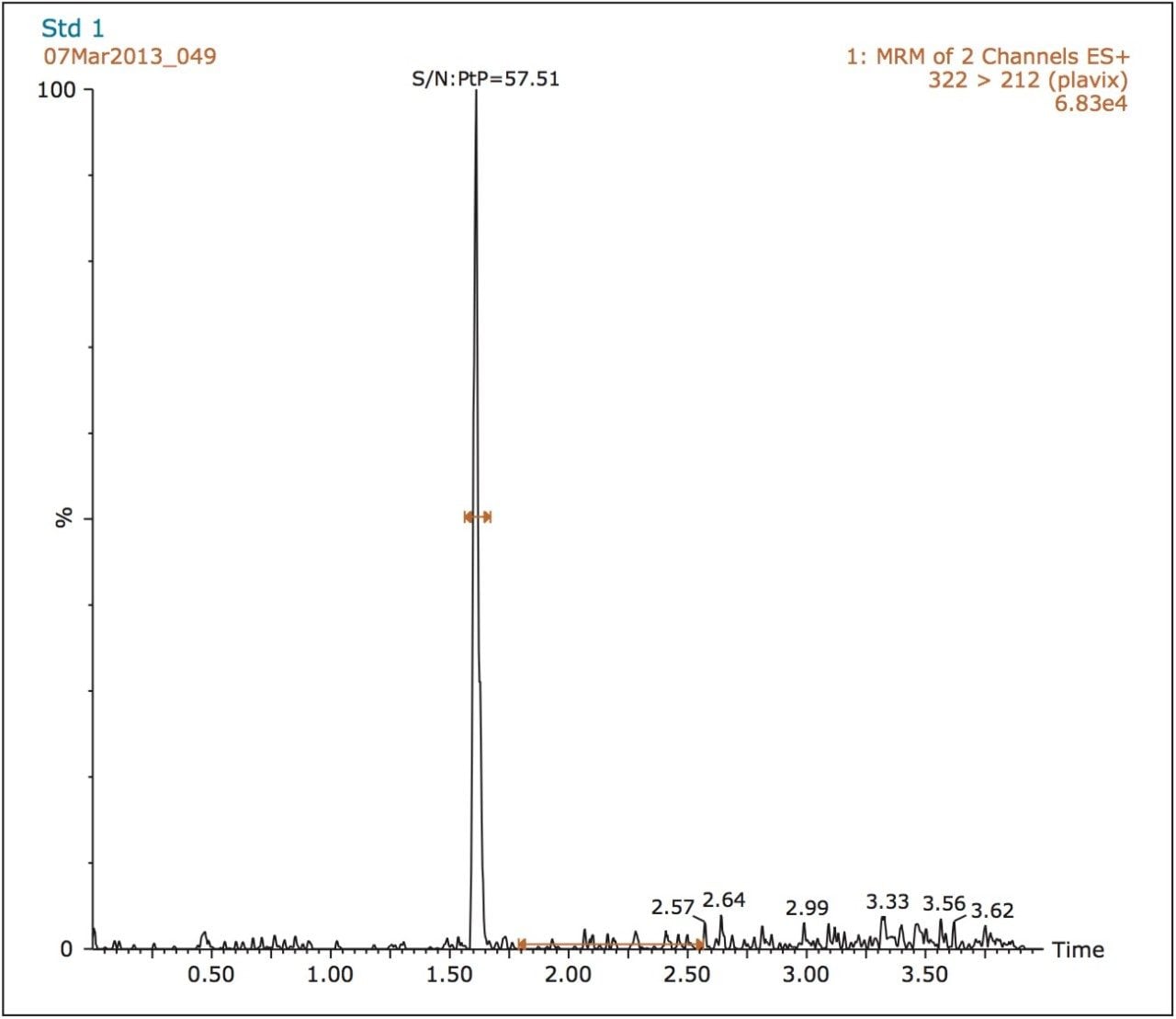
Clopidogrel eluted with a retention time of 1.6 minutes, as shown in Figure 2. Data show that the peak produced by the chromatography system was symmetrical and narrow with a width measured at peak base of three seconds. There is also very little background noise present using this method, facilitating a signal-to-noise value of approximately 60:1 for the lowest standard, as shown in Figure 2.
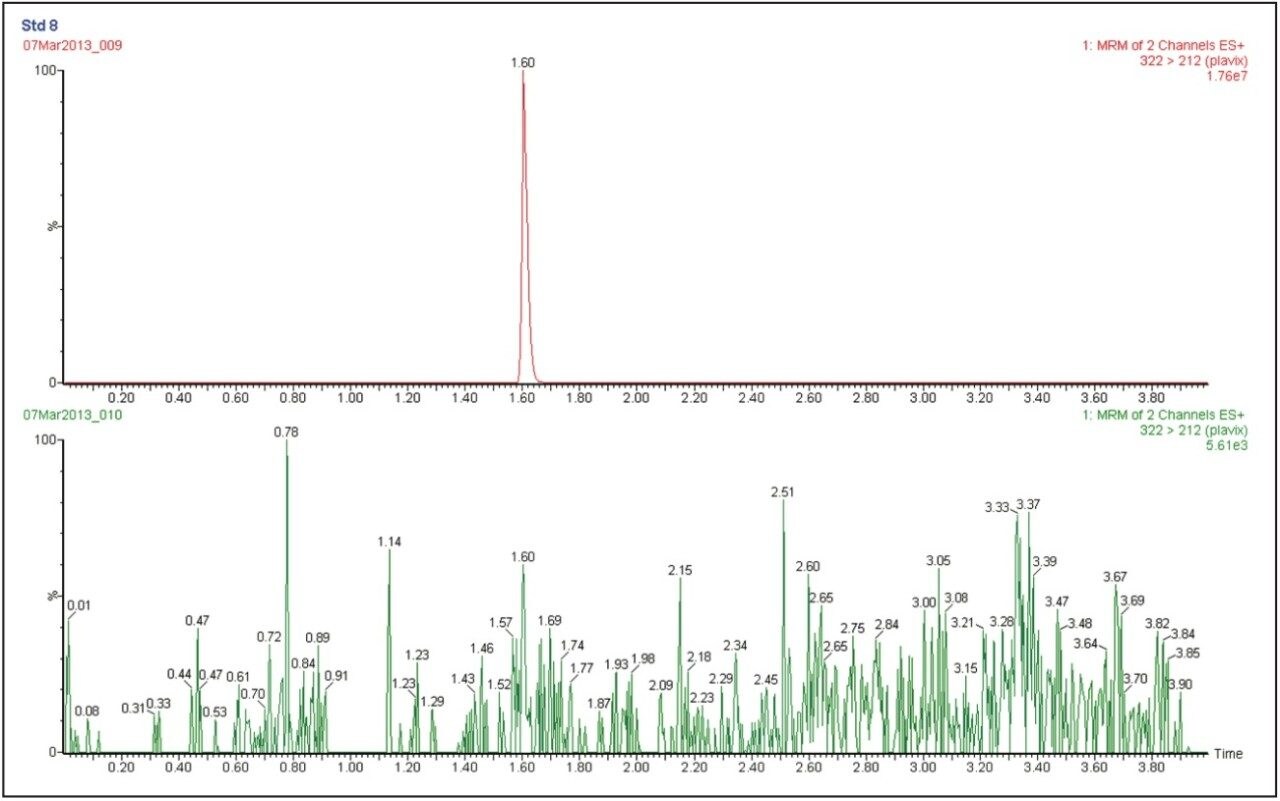
The data in Figure 3 illustrates the injection of an extracted plasma blank injection immediately following the analysis of a 5000 pg/mL standard. There is no carryover in the blank chromatogram, critically important for any bioanalytical method.
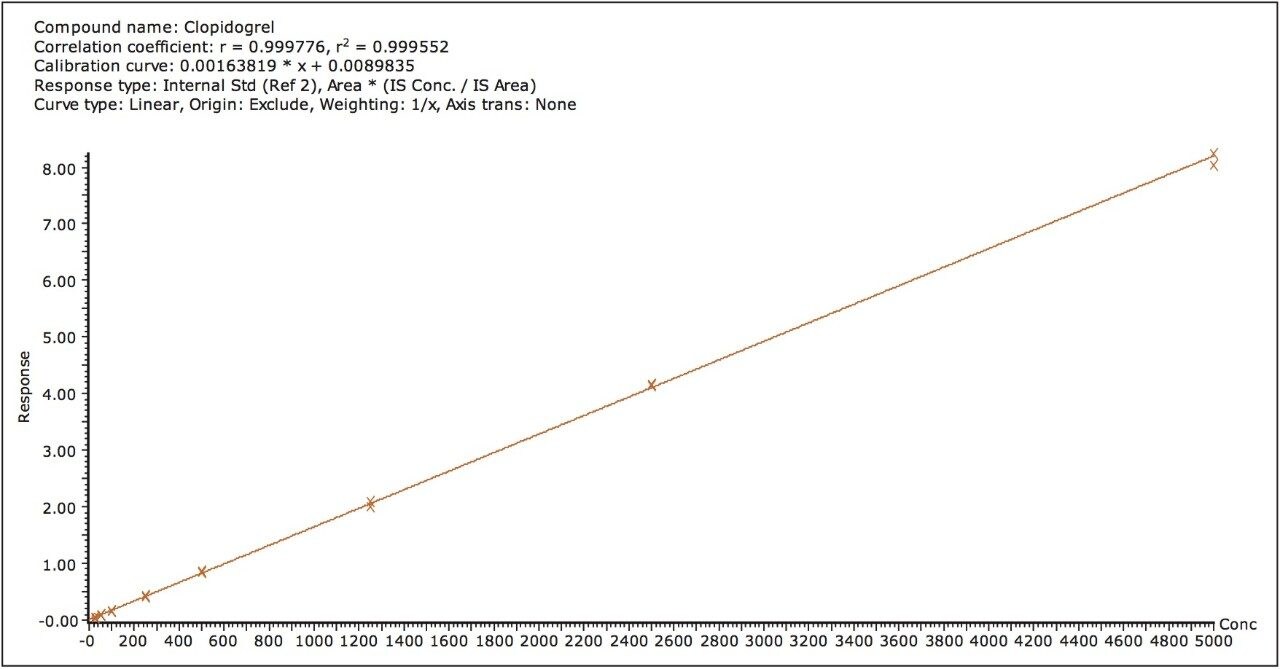
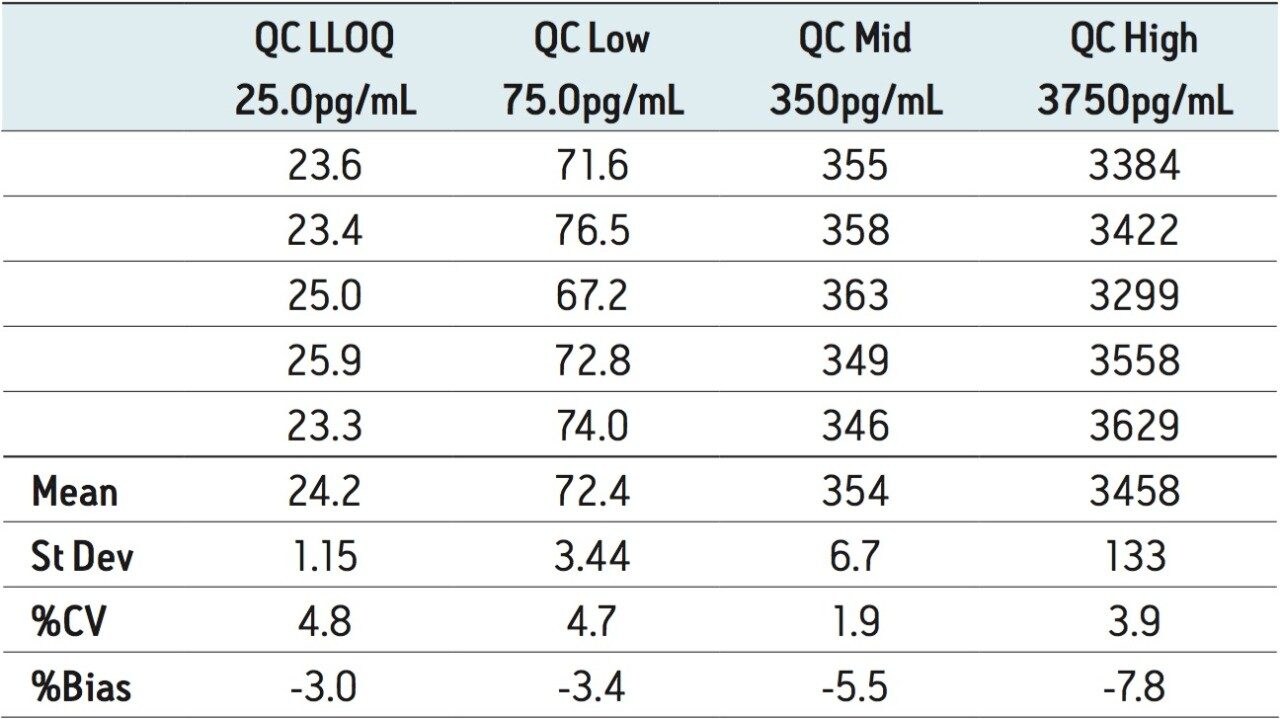
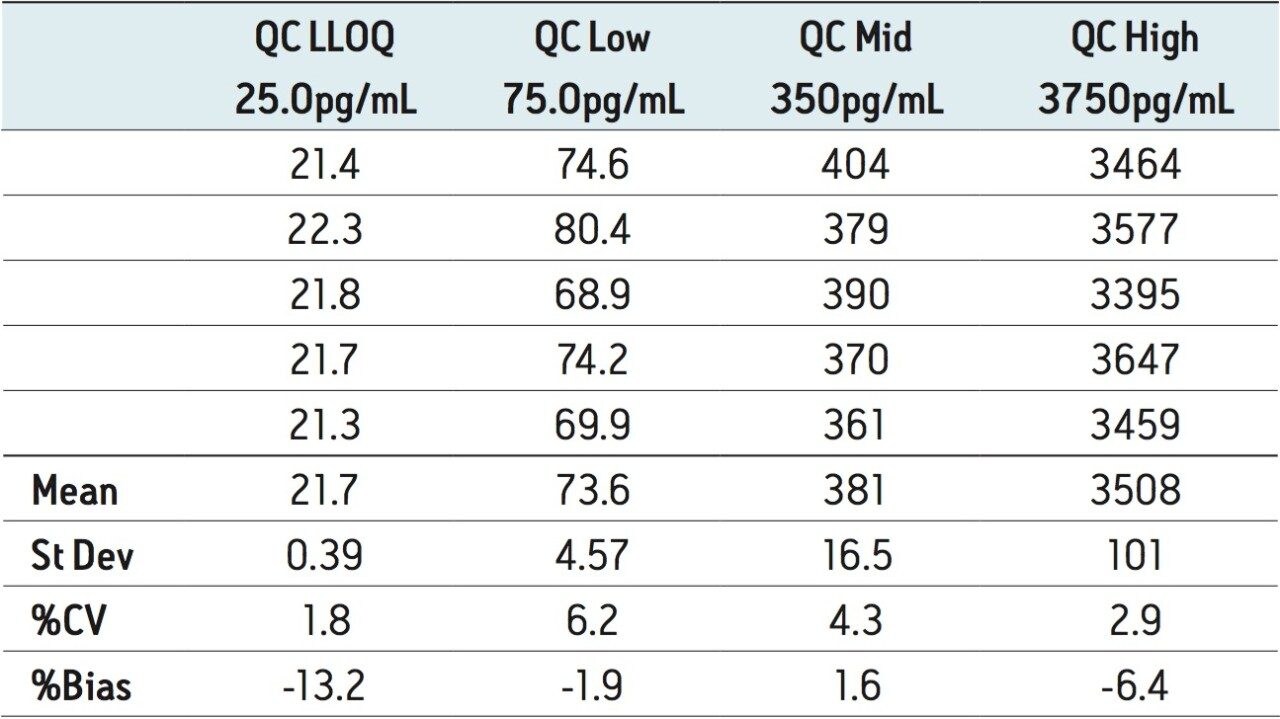
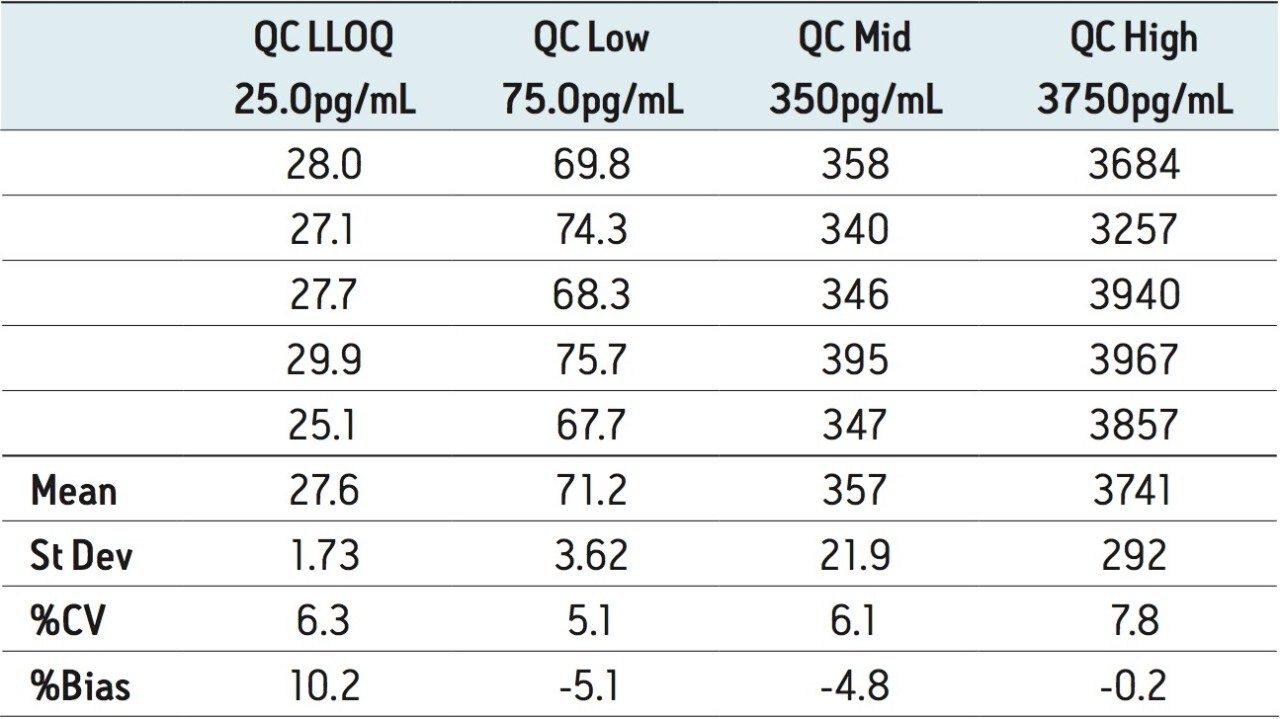
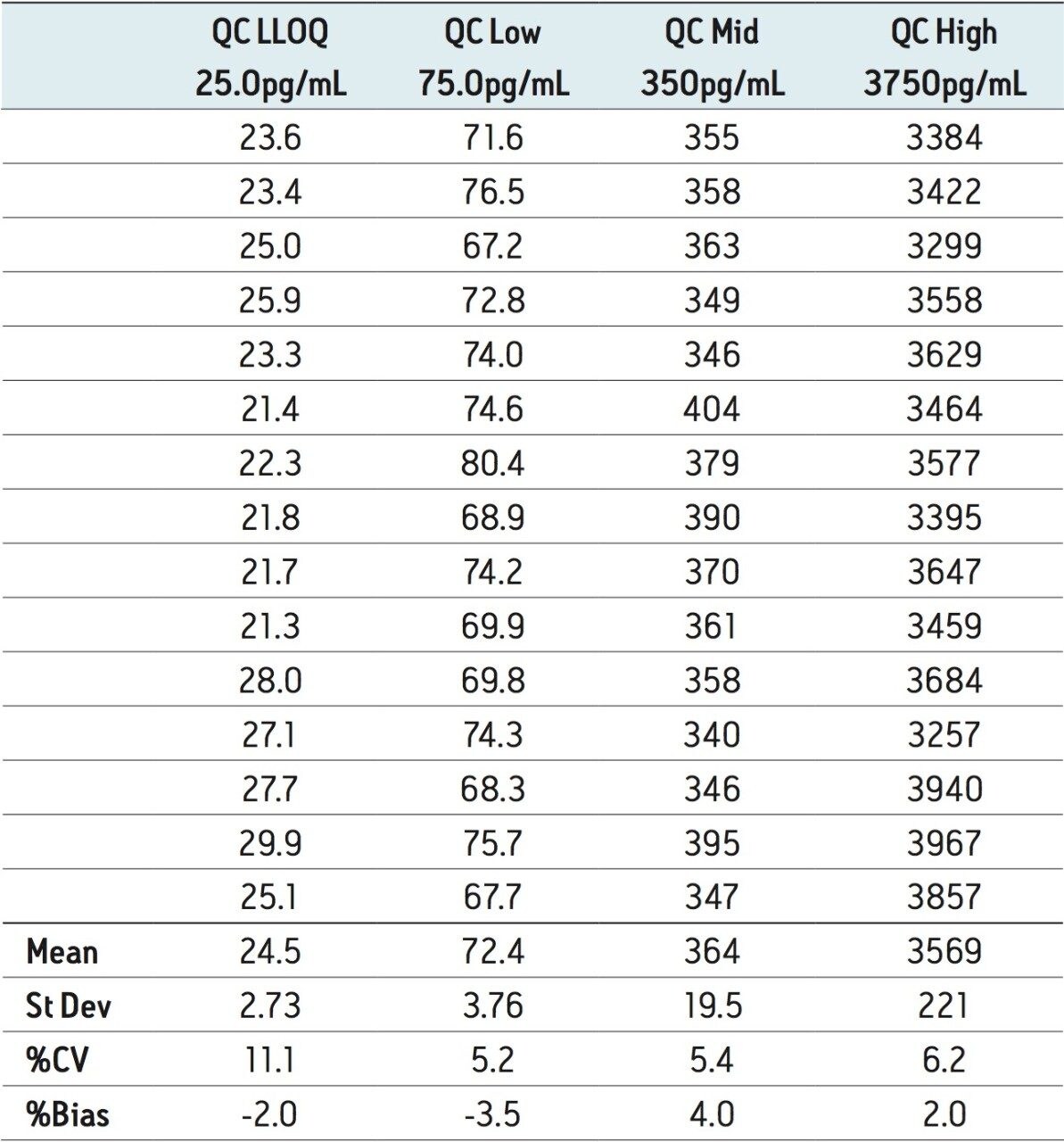
The assay was validated with separate accuracy and precision batches on three consecutive days ranging from 25 to 5000 pg/mL. A typical calibration obtained for the assay is shown in Figure 4, whereby the correlation coefficient ranged between 0.996 and 0.999 using a 1/x weighting linear regression. The intra-day precision and accuracy validation data are displayed in Tables 1 through 3. The validation data show that the coefficient of variation ranged from 1.8% to 6.3% for the 25.0 pg/mL LLOQ, with a bias between -13.2% and 10.2%. The high QC (3750 pg/mL) coefficient of variation ranged from 2.9% to 7.8% with a bias between -7.8% and -0.2%. The inter-day precision and accuracy data are displayed in Table 4. The coefficient of variation was determined to be 11.1% for the 25.0 pg/mL LLOQ with a bias of -2.0%. For the high QC (3750 pg/mL), the coefficient of variation was determined to be 6.2% with a bias of 2.0%.
720004676, May 2013