This application note highlights the importance of selecting an optimal column stationary phase, by demonstrating changes in selectivity of various types of samples including synthetic mixtures, forced degradation reactions and natural product extracts, across different columns.
Column selection for chromatographic analysis is an important step in method development that can have significant consequences to the effectiveness of the separation. If the wrong column is chosen, the length of time and effort to develop and optimize the separation may be unnecessarily long. Many labs have limited column selection and may base their methods on one core column chemistry, such as a conventional endcapped C18 column. However, with advances in column technology, there is an increase in the availability of different base particles and ligand chemistries to screen for alternate selectivity and achieve improved separations.
This application note highlights the importance of selecting an optimal column stationary phase, by demonstrating changes in selectivity of various types of samples including synthetic mixtures, forced degradation reactions and natural product extracts, across different columns. Sample screening across multiple column chemistries was automated using an ACQUITY UPLC H-Class System with a column manager, and shifts in the compound elution order were monitored by UV and mass spectrometric detection. Proper column selection is essential in quickly establishing an effective method and minimizing the need for further extensive method development and optimization.
|
Mobile Phase: |
A: water with 0.1% formic acid, B: acetonitrile with 0.1% formic acid |
|
Gradient: |
2 to 98% B over 5 minutes, hold for 1 minute, re-equilibrate at 2% B |
|
Detection: |
UV at 254 nm |
|
SQD: |
ESI+ mode, mass range 100-600 amu |
|
Needle Wash: |
90:10 acetonitrile:water |
|
Sample Purge: |
90:10 water:acetonitrile |
|
Seal Wash: |
50:50 methanol:water |
|
Flow Rate |
0.8 mL/min |
|
Column Temp.: |
30 °C |
|
Injection Volume: |
2 μL |
|
Columns: |
ACQUITY UPLC, 2.1 x 50 mm, 1.7 – 1.8 μm |
|
Stationary Phases: |
BEH C18, part number 186002350 BEH Shield RP18, part number 186002853 CSH C18, part number 186005296 CSH Fluoro-Phenyl, part number 186005351 CSH Phenyl-Hexyl, part number 186005406 HSS Cyano, part number 186005986 HSS PFP, part number 186005965 HSS T3, part number 186003538 |
Empower 3 CDS
Nadolol and 3,4-dihydroxy phenylacetic acid: Samples representative of synthetic reaction products were prepared by acetylating 10 mg of each compound. Compounds were first dissolved in pyridine and dichloromethane. Acetic anhydride was added, the reaction was heated to 40 °C and stirred for one hour. Samples were concentrated by rotary evaporation and resuspended in acetonitrile for injection.
Ziprasidone base degradation: A 1 mg/mL solution of ziprasidone was prepared in methanol. To this was added 0.1N NaOH and the reaction was heated at 80 °C for two hours. The reaction was neutralized with 0.1N HCl and transferred to a vial for injection.
Ashwagandha root: 1200 mg of ashwagandha root (Withania somnifera) was extracted with 2 mL of methanol, stirring at room temperature overnight. The extract was centrifuged at 12,000 rpm for 10 minutes to remove any particulates prior to injection.
Selection of the proper column early in the method development process is crucial to obtain an optimal separation. If a separation is developed on a generic column chemistry (perhaps based on column availability in the lab) the chromatography may not be ideal, resulting in further method development that may be unnecessarily complicated and highly time consuming. Instead, if several different column stationary phases are screened to rapidly identify a column providing the best separation, subsequent method development may be minimal or even unnecessary. To maximize the selectivity differences of comparative separations, columns with very different stationary-phase properties can be identified using the Waters Column Selectivity Chart (www.waters.com/selectivitychart). Sample screening on various columns is streamlined and automated using the ACQUITY UPLC H-Class System with a column manager and Empower 3 Software.
A variety of samples were prepared to examine selectivity differences between columns. Although pH is a great effector of peak shape and selectivity in method development, only the low-pH method condition is compared here to clearly monitor the effects of changing only the column stationary phase. Compound identification for every peak in each sample was not performed due to the complexity of the samples, instead, the base peak mass of the major peaks were used to track changes in selectivity.
The base degradation sample of ziprasidone was analyzed on a number of different column chemistries to examine the effects of the base particle and bonded phase chemistry on the separation (Figure 1). Significant changes in elution order and retention of this sample are seen with different column chemistries. The ACQUITY UPLC BEH (Ethylene-Bridged Hybrid) C18 Column is a very robust column frequently used in UPLC. In this case, the BEH column provides an adequate separation, but lacks baseline resolution between the peaks 1 and 2. The CSH C18 column has the same ligand but the chromatography shows a completely different elution order and increased resolution between all peaks, due solely to the applied charge on the surface of the CSH particle.
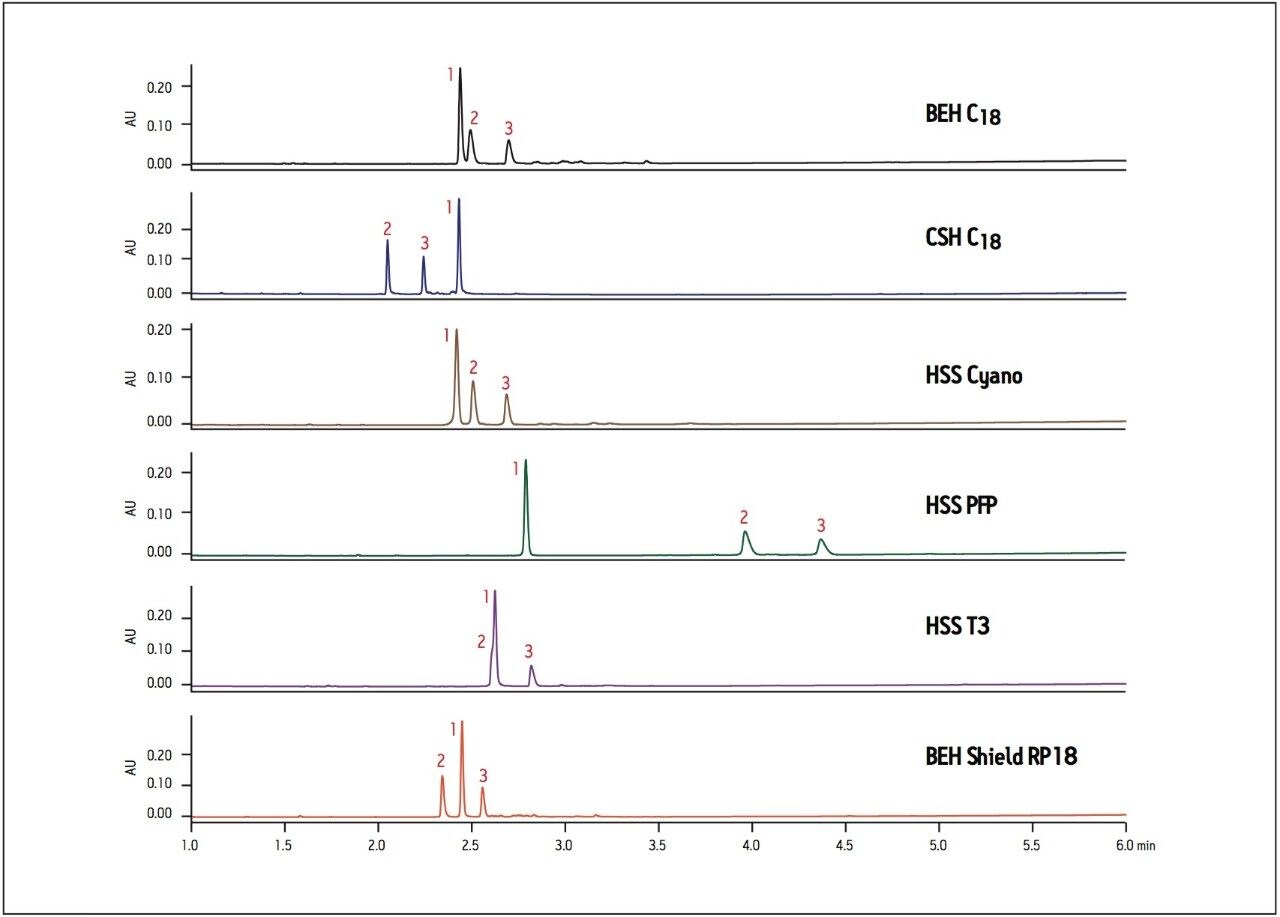
The HSS (High-Strength Silica) Cyano column provides similar retention but increased resolution compared to the BEH C18 column, whereas the HSS PFP column separation shows increased retention of all components, particularly minor components 2 and 3. The HSS T3 column has a C18 ligand on an HSS particle but has lower ligand density resulting in a slight increase in retention and change in elution order compared to BEH C18, with a co-elution of peaks 1 and 2. Finally, chromatography on the BEH Shield RP18 column shows a change in elution order compared to BEH C18 with baseline resolution of peaks 2 and 3. There is also less retention of all components due to the fewer interactions with the shielded silanol groups on the base particle. Overall, the ziprasidone base degradation sample shows very different selectivity when analyzed on a variety of column particles and ligands. Initial use of a BEH C18 or HSS T3 column would require additional method optimization to fully resolve the components. By rapidly screening a wide range of columns and selecting a column that demonstrates good resolution early, the need for further method development in such cases can be avoided. In this example, the CSH (Charged-Surface Hybird) C18 column may ultimately be chosen for its sharp peak shapes and improved resolution of impurities away from the API peak.
Synthetic reaction mixtures may contain unreacted starting materials, reagents, reaction side-products and target compounds that require separation. In situations where it may be important to identify or resolve targeted components or product impurities, proper assessment of the separation on various column stationary phases is essential. Changes in selectivity may provide increased resolution of the targeted peak of interest, facilitating identification and purification should the separation be scaled up to a larger diameter column. The separation of acetylation reaction products of 3,4-dihydroxy phenylacetic acid is shown in Figure 2, where the CSH C18 column shows shifts in retention time and elution order compared to the BEH C18 column. These shifts are due to the effect of the charged surface of the CSH particle on ionizable analytes in the sample. The Fluoro-Phenyl ligand on the CSH particle shows elution order differences and overall less retention compared to the CSH C18 and BEH C18 columns. Since some analytes in this reaction mixture have aromatic properties, interactions between the analytes and a Phenyl-Hexyl ligand on a CSH particle results in shifts in elution order and altered selectivity. Interactions between the analyte and the short cyano ligand on the HSS Cyano column results in overall reduced retention of hydrophobic analytes and different selectivity compared to all other columns screened.
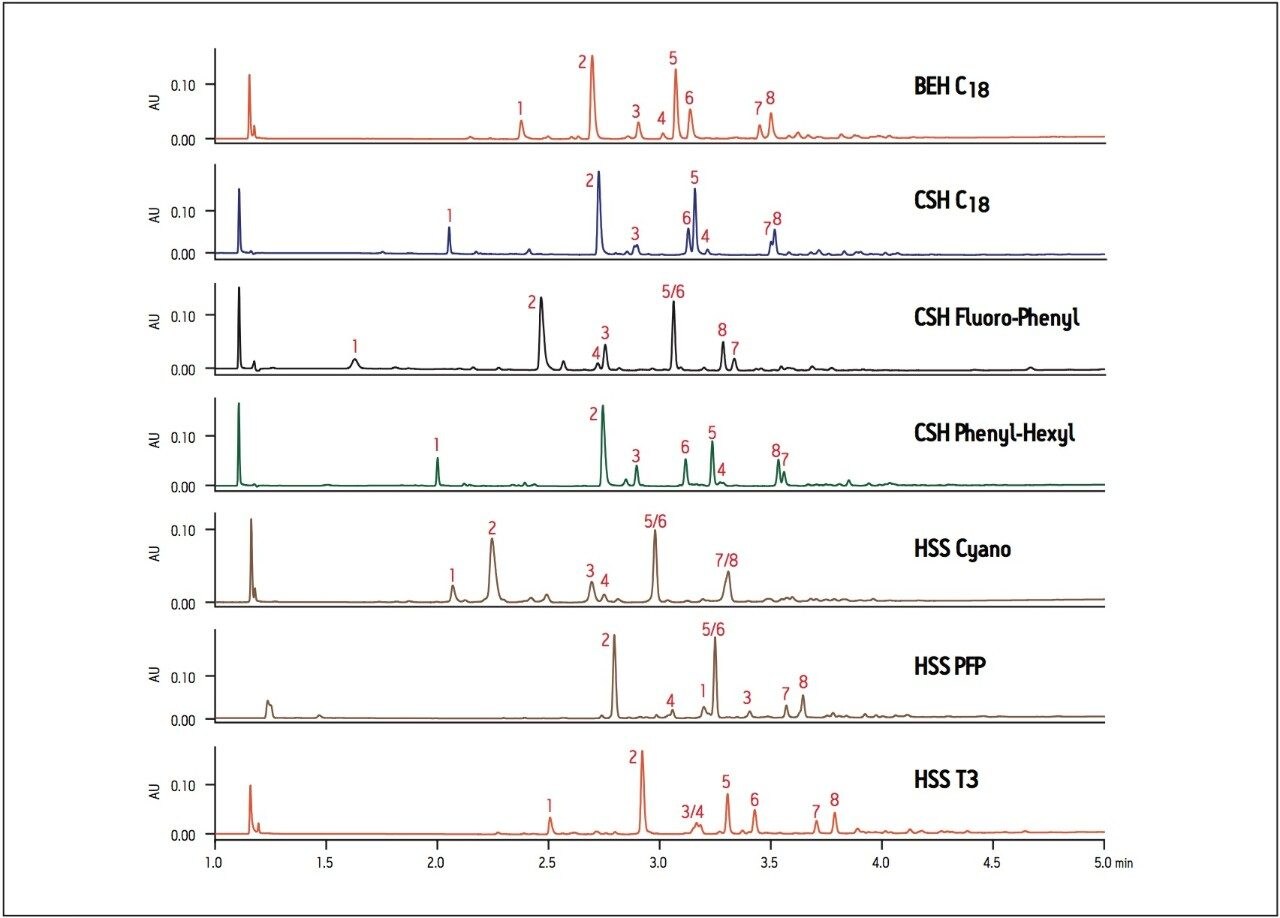
The HSS PFP column has the same fluoro-phenyl ligand as the CSH Fluoro-Phenyl column but is bonded to a HSS particle instead of the CSH particle. The difference in the properties of the base particles results in very different elution order and retention between the two columns. Finally, the HSS T3 column has a similar elution order to the BEH C18 column, but gives improved resolution between peaks 5/6 and 7/8. In this example, the BEH C18 column gives adequate resolution for all 8 compounds, but if we focus on peak 6 as the target peak of interest, the best resolution and peak shape is obtained on the HSS T3 column.
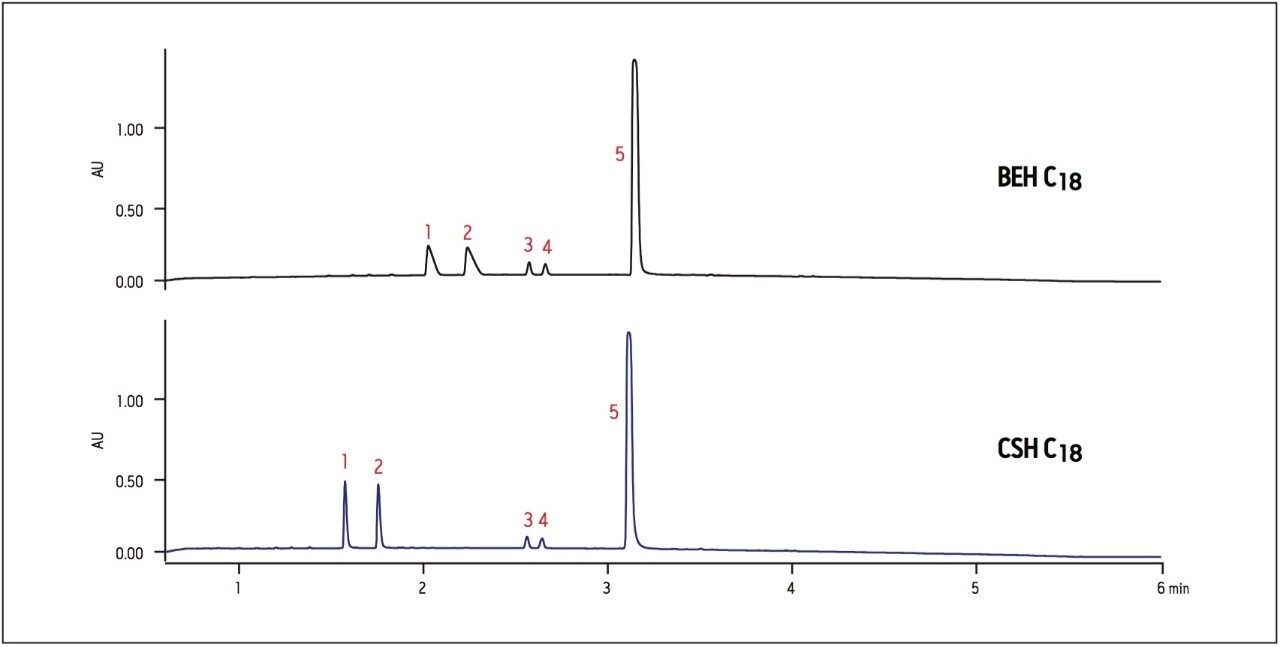
Another important consideration when selecting a column is the loading capacity of the stationary phase. While basic compounds often have better loading and peak shape at high pH on compatible hybrid particle columns such as BEH or CSH, they tend to have worse peak shape and loading on traditional C18 columns in low-ionic-strength mobile phases, such as formic acid.2 However, a CSH column can provide better loading of basic compounds at low pH using formic acid, resulting in sharper peak shapes and enhanced sensitivity of detection. Loading limitations are demonstrated in the analysis of acetylation products of nadolol, where the reaction products labeled as peaks 1 and 2 show overloaded peak shape on the BEH C18 column (Figure 3). By contrast, these peaks are considerably sharper, with enhanced loading and sensitivity on the CSH C18 column. At low pH, greater sensitivity and peak shape for these basic compounds allows faster identification of impurities on the analytical scale, and facilitates isolation of desired peaks at the preparative scale.
When screening natural product extracts that contain many different types of compounds, it is particularly important to screen a wide selectivity range of columns. Selectivity can vary greatly when running extracts on various column chemistries and identification of minor components from complex crude extracts may be easily missed without proper screening. In Figure 4, the chromatographic profile of an extract of ashwagandha root is compared on four different column chemistries that were identified as having a wide selectivity range using the Waters Column Selectivity Chart (www.waters.com/selectivitychart).
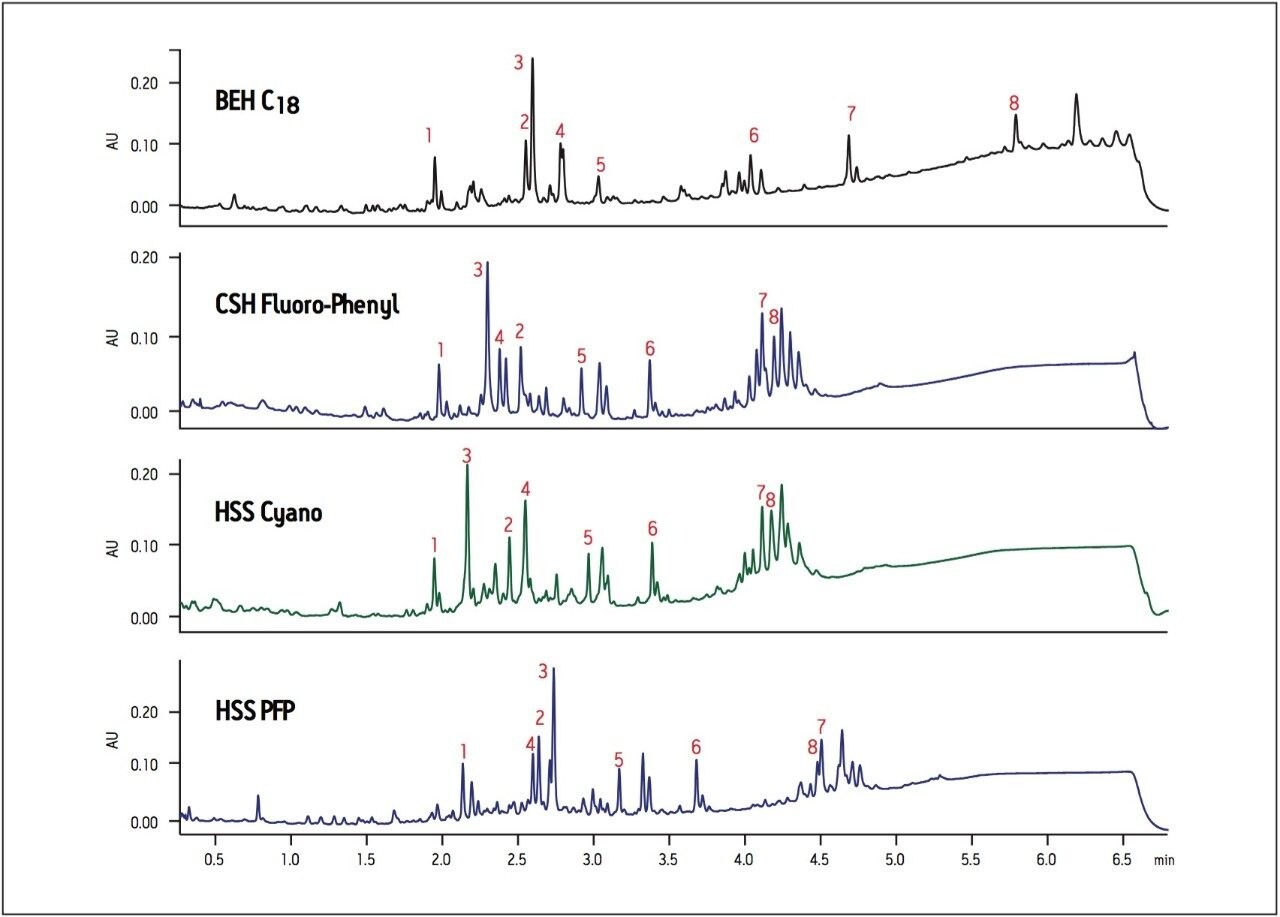
The BEH C18 column shows increased retention for hydrophobic compounds compared to the CSH Fluoro-Phenyl, HSS Cyano or HSS PFP columns (Figure 4). There are also considerable peak elution order and retention differences observed across all columns, especially in the regions of peaks 2 to 4. Note again the significant difference in selectivity between the CSH Fluoro-Phenyl and HSS PFP columns. Although they both have the same ligand chemistry, they display significantly different chromatography due to the differences in base particle, making these two columns particularly good orthogonal choices for column screening. In this example, peak 7 is clearly resolved using the BEH C18 column, whereas the separation and identification of peak 2 is more readily achieved using the HSS Cyano column, thus illustrating the utility of screening across different column chemistries. Early screening of extracts using columns with a wide range of selectivity facilitates rapid identification of minor components in complex mixtures by providing a better chance of resolving peaks of interest and enabling more accurate compound identification using mass spectrometry.
Proper column selection considering appropriate base particle and bonded-phase chemistry is an important tool in rapidly developing methods for effective separations. Poor column choice early in the development of a new method can result in costly and unnecessary secondary optimization experiments. With advances in column technology, there are increasing choices of columns with different base particles and ligands to provide optimal chromatography. For the separation of components in any matrix, sample screening across a wide range of column chemistries should be considered. Columns with diverse chemical properties can be easily selected using the Waters Column Selectivity Chart. Screening of samples across columns is automated using the ACQUITY UPLC H-Class System with a column manager and Empower 3 Software. Using these tools, rapid screening on a variety of columns can be performed for each sample, resulting in faster and more efficient method development with improved separations.
720004353, June 2012