
This application note describes the application of ultra sensitive detection to minimize the impact of matrix effects during analysis of 81 pesticide residues in a range of food products and also the use of a novel MRM acquisition mode that allows direct monitoring of the matrix background in each sample injected.
One of the biggest challenges in ensuring the safety of our food supplies is the measurement of hazardous ultra trace level components in the presence of a highly complex sample matrix. For the analysis of pesticides in food matrices, increased use of liquid chromatography systems, coupled with tandem quadrupole mass spectrometers has allowed progress in reducing the problems caused by the sample matrix. However, difficulties remain when trying to discriminate against matrix components that exhibit similar physiochemical properties. Unawareness of these difficulties in each unique sample can lead to poor quality results, and can impact a laboratory’s performance and reputation.
Understanding the matrix challenge of each injected sample is clearly beneficial as is the ability to monitor changes in the sample matrix between samples and batches. This capability can lead to the continuous improvement of analytical quality in the laboratory. Conventional LC tandem quadrupole systems do not allow the direct monitoring of the sample matrix during high sensitivity MRM quantitation and it is only recently with the newest generation of instruments that this has become possible.
Problems caused by the sample matrix can include disruption to chromatography, increased chemical noise, and most notably, ionization suppression.1-4 In highly complex matrices such as herbs and spices, these problems can be found in combination to make determination of pesticide residue concentration very difficult.
In addition to problems caused by the sample matrix, there are also pesticides that, by nature, are more difficult to analyze using LC-MS/MS due to a poor (relative) response factor. Successful analysis of these compounds to the regulatory concentration limits is difficult when considering the practicality of increasing sample amount and the balance of extracted matrix concentration. A much more practical solution is to use increased instrument sensitivity to maximize performance at these required concentrations. Also, if enough sensitivity is available, then the reduction of matrix concentration injected onto the system becomes possible.
Described here is the application of ultra sensitive detection to minimize the impact of matrix effects during analysis of 81 pesticide residues in a range of food products. Also described is the use of a novel MRM acquisition mode that allows direct monitoring of the matrix background in each sample injected.
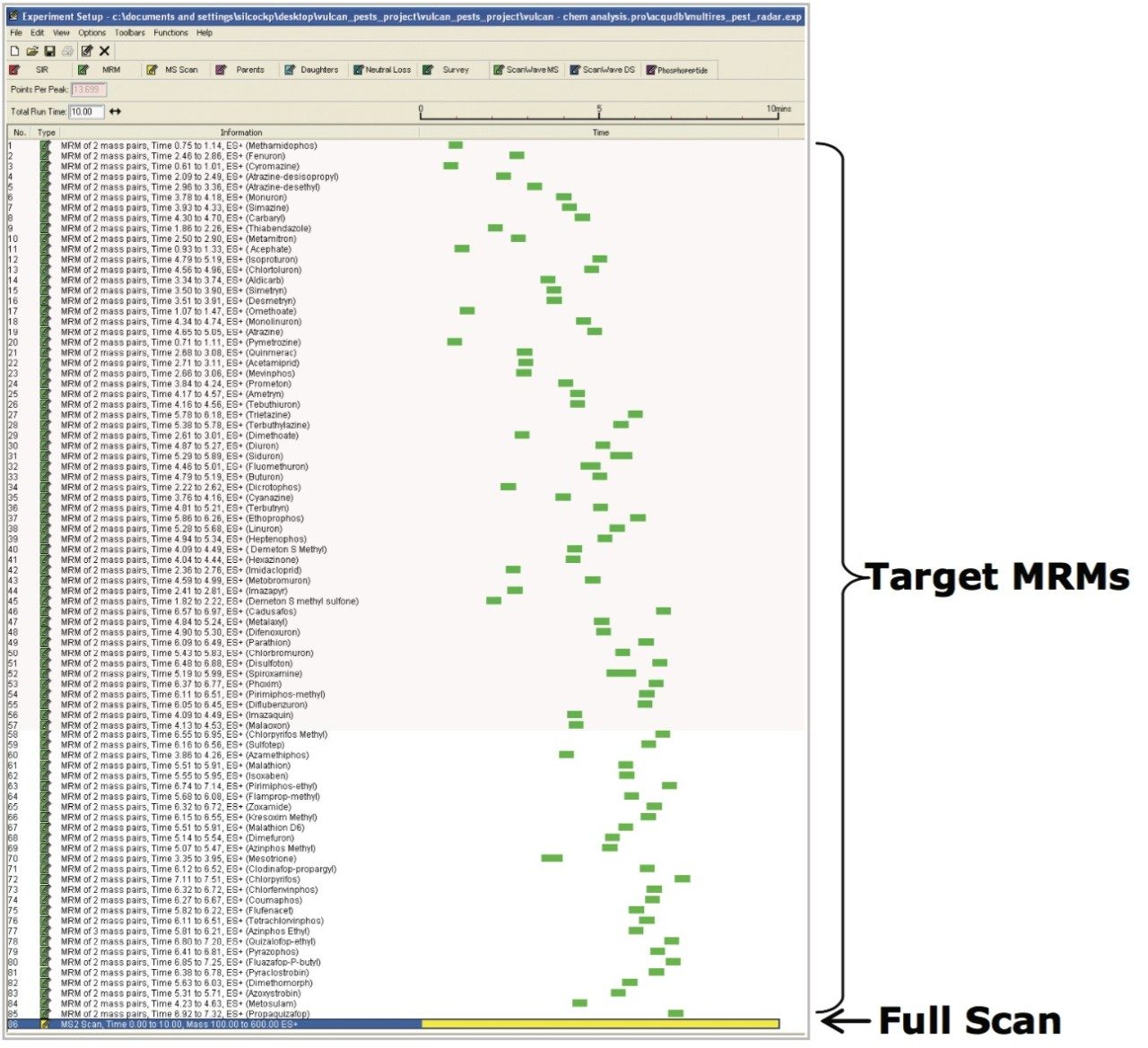
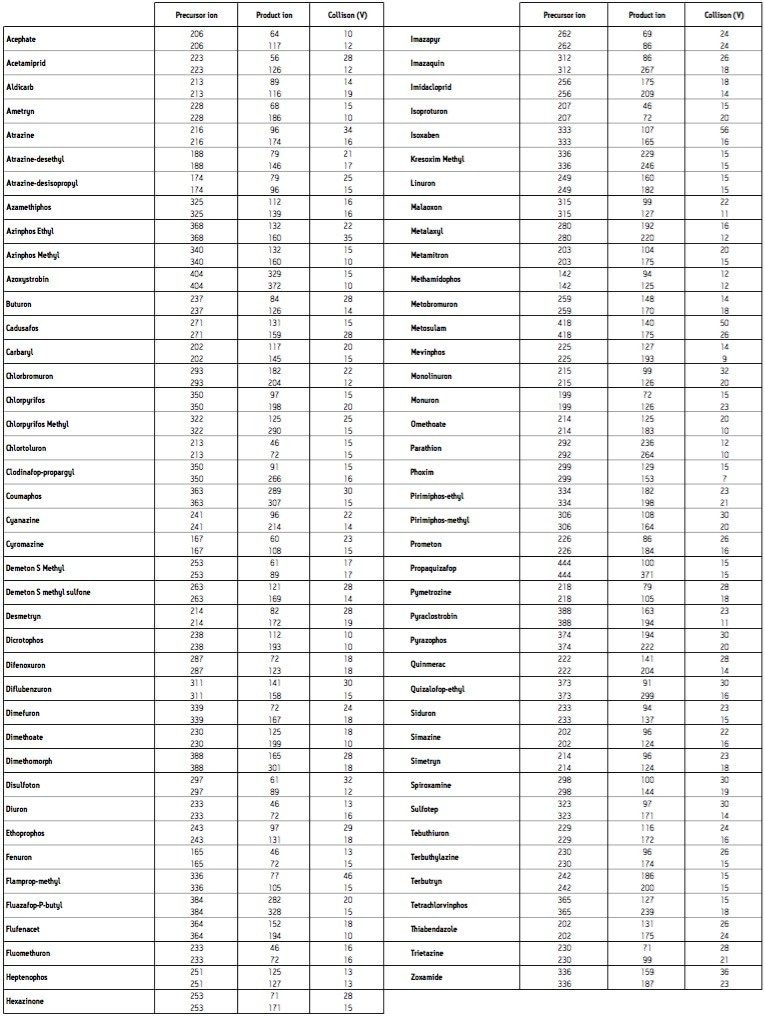
Quanpedia generated MRM parameters (a full MRM list can be found in Appendix 1) were used as the basis of RADAR-enabled mass spectrometer acquisition method. RADAR is an information-rich acquisition approach that allows measurement of target analytes with precision in MRM mode, while simultaneously scanning the background for all other components.
Figure 1 shows a RADAR-enabled mass spectrometer acquisition method with time scheduled MRMs for target pesticides and a simultaneous full scan (MS2) acquisition.
Waters DisQuE (EN 15662:2008) Extraction Kit (QuEChERS) was used to prepare spiked extracts of grape, avocado, marjoram, and ginger. Sample matrix concentrations were 1g/mL for grape and avocado and 0.1 g/mL for marjoram and ginger. The final acetonitrile extracts from QuEChERS were diluted 10x into mobile phase and 10 μL were injected onto the analytical system (referred to as original sample). Subsequent dilutions of this were then made to reduce matrix effects.
|
LC system: |
ACQUITY UPLC |
|
Column: |
ACQUITY BEH C18 100 mm x 2.1 mm, 1.7 μm |
|
Mobile phase A: |
0.1% HCOOH in H2O |
|
Mobile phase B: |
0.1% HCOOH in MeOH |
|
Run time: |
10.00 min |
|
Time (min) |
Flow (mL/Min) |
%A |
%B |
|---|---|---|---|
|
- |
0.5 |
90 |
10 |
|
0.25 |
0.5 |
90 |
10 |
|
7.75 |
0.5 |
2 |
98 |
|
8.5 |
0.5 |
2 |
98 |
|
8.51 |
0.5 |
90 |
10 |
|
MS system: |
Xevo TQ-S |
|
Ionization mode: |
ES positive |
|
Capillary voltage: |
0.60 kV |
|
Source temp: |
130 °C |
|
Desolvation temp: |
650 °C |
|
Cone gas flow: |
150 L/hr |
|
Desolvation gas flow: |
1200 L/hr |
European Union (EU) regulations to control pesticide exposure from food consumption are among the toughest in the world. In order to import food and food commodities into Europe, the level of pesticide contamination must be below the stated maximum residue limits (MRLs) for that product.5 Confirmation of positive results requires good quantitative performance well below these concentrations, which can be very challenging in more complex matrices.
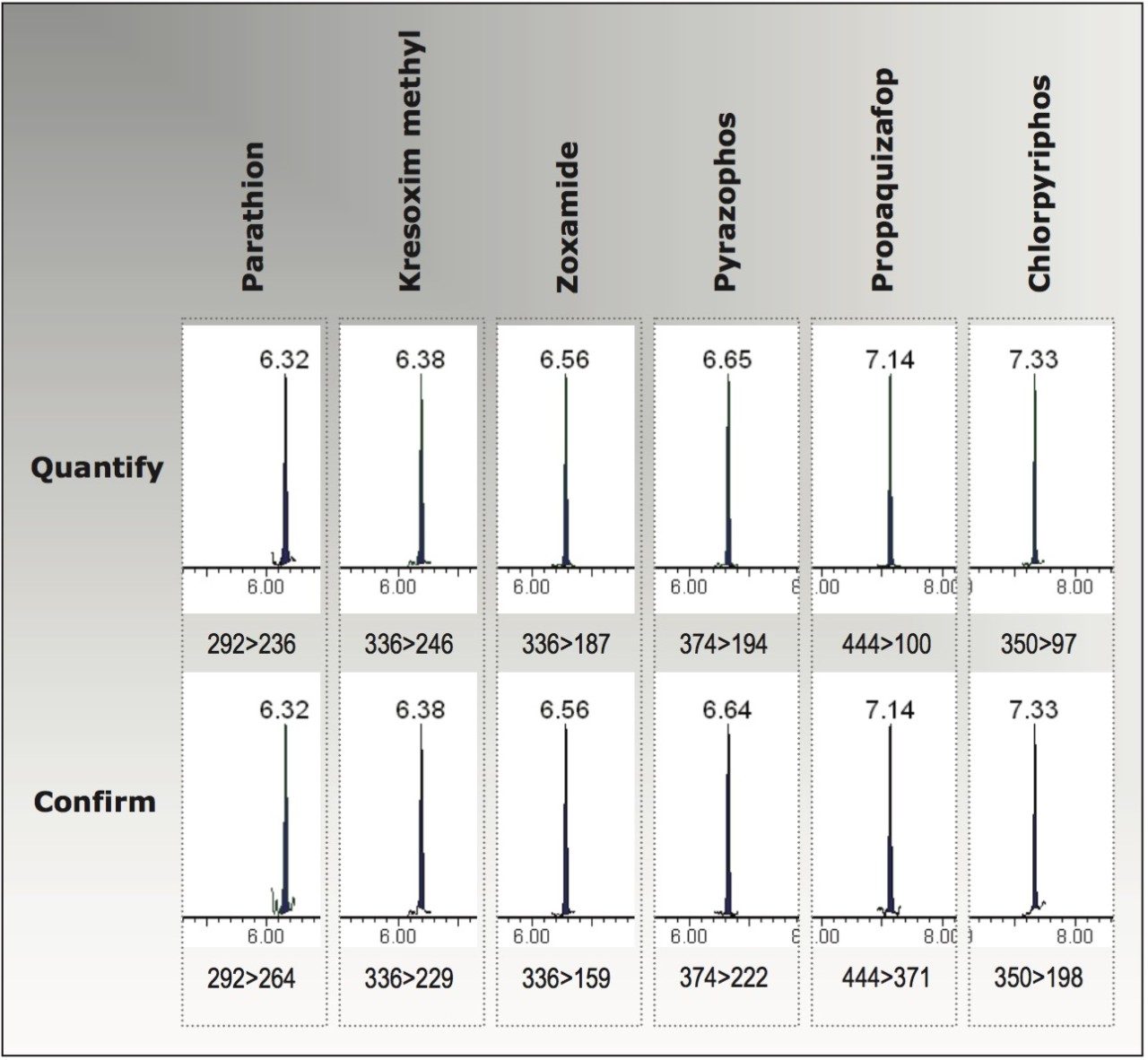
Figure 2 shows a selection of extracted MRM chromatograms for pesticides spiked into avocado at 0.005 mg/kg. Quantitative and confirmatory transitions are both detected at this level, which is 10x below the European MRL (except zoxamide, which is 4x below). This includes parathion, which has a relatively poor response factor when analyzed using electrospray ionization. Comfortable quantitation of pesticides at these low concentrations allows high confidence when reporting results around maximum residue limits.
Each sample analyzed had full scan data available along with the MS/MS data. This was due to the RADAR functionality of the Xevo TQ-S being enabled. These data were used to monitor the complexity of the sample matrix background in each sample.
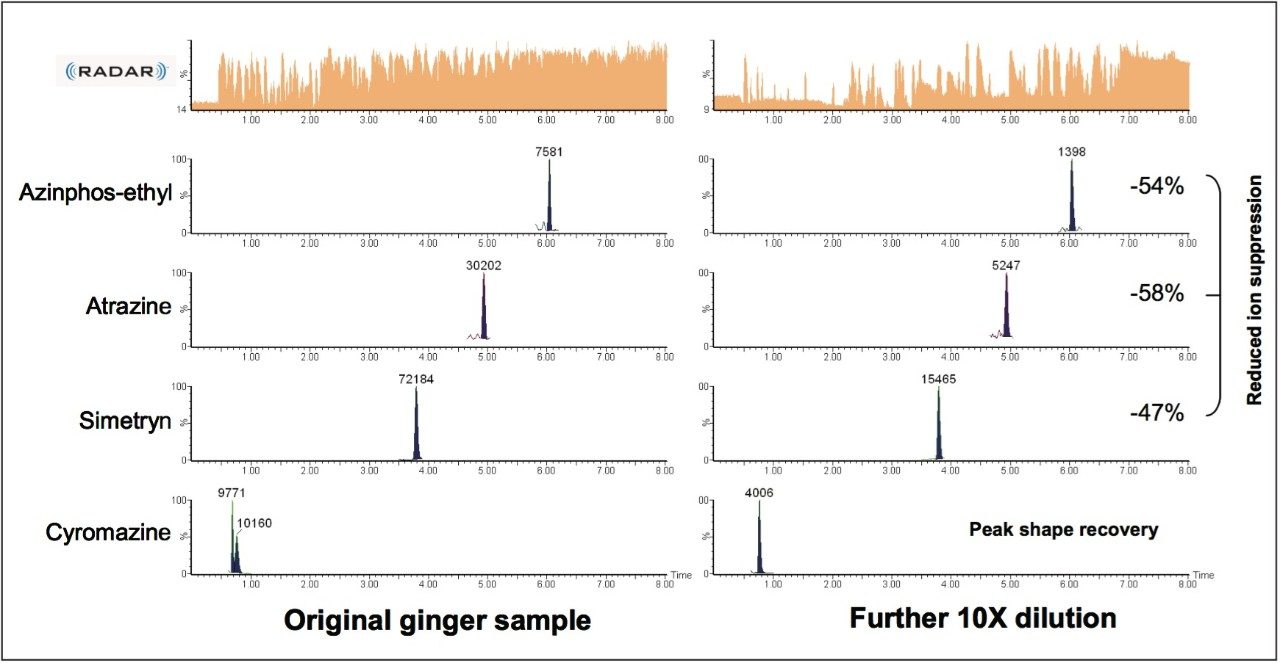
Differences in the co-extracted background for grape, avocado, marjoram, and ginger were observed by plotting the base peak intensity (BPI) chromatogram. For ginger and marjoram, 10x less sample was extracted using QuEChERS to give a 0.1 g/mL matrix, as opposed to the usual 1 g/mL matrix for grape and avocado. This is due to the extremely high complexity of the sample matrix, as well as to aid extraction of these drier samples. Figure 3 shows base peak intensity (BPI) chromatograms overlaid with MRM chromatograms for pesticides spiked at 1.0 x 105 g/kg for each matrix.
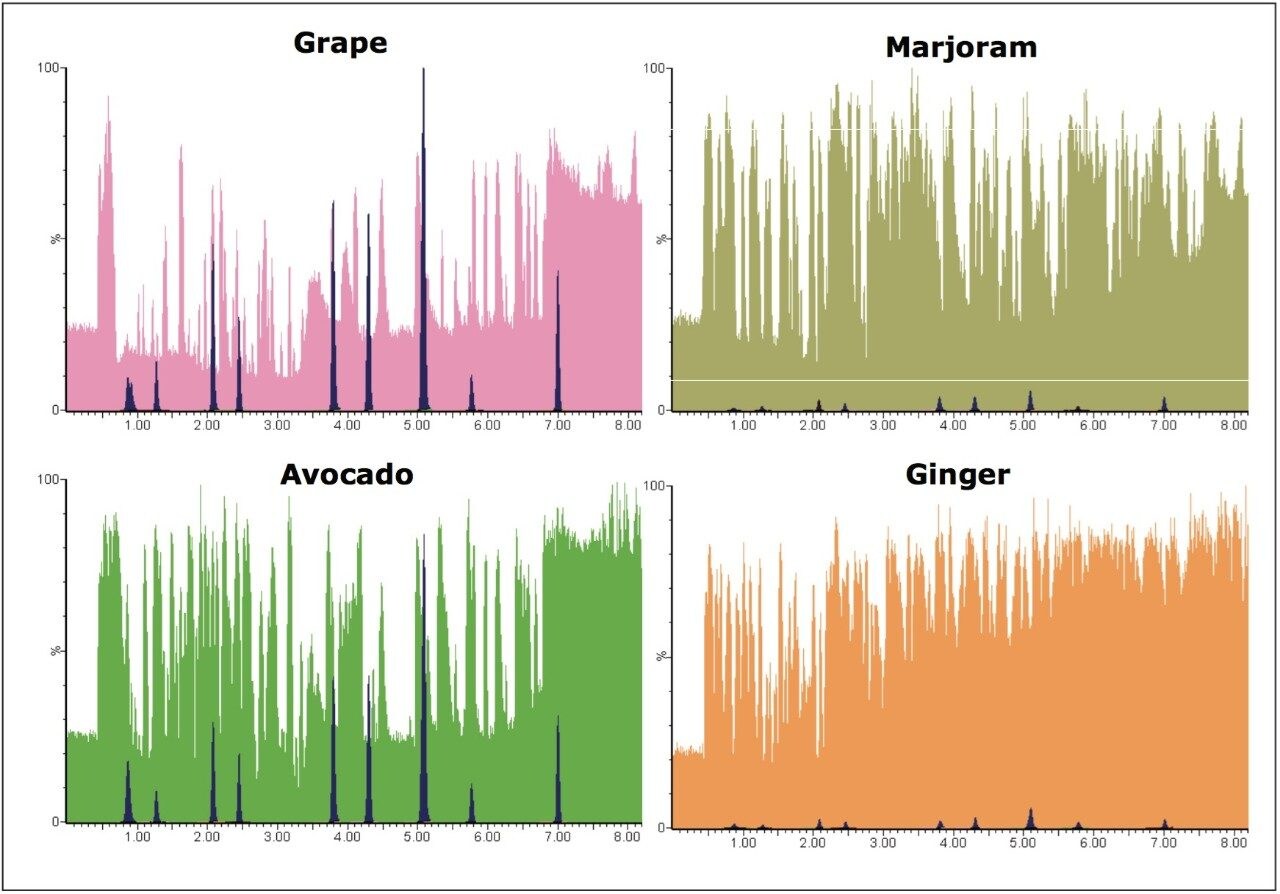
Despite the reduction in matrix concentration, the ionizable background is high in marjoram and ginger samples, compared with grape and avocado; as a consequence, the likelihood for analyte ion suppression (and enhancement) may be higher for these types of samples.
With simultaneous full scan it is also possible to observe specific components that co-elute with target analytes. Figure 4 shows BPI and MRM mass chromatograms for a grape sample spiked with dimethoate at 0.01 mg/kg. Full scan spectra from the elution region of dimethoate were combined and the most intense ion from the mass spectrum extracted into another chromatogram (XIC), revealing a discrete peak that co-elutes with dimethoate, as shown in Figure 4.
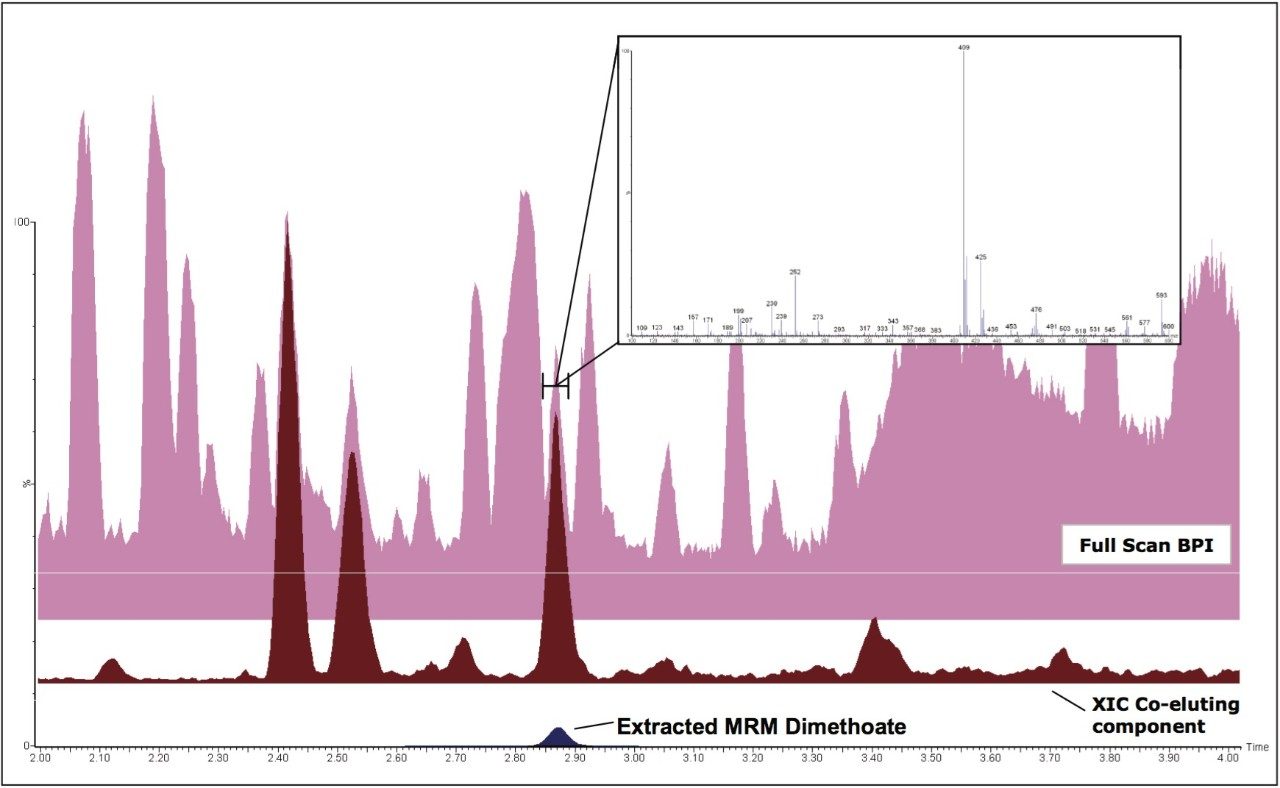
If significant problems are observed with this or any other components in the matrix, the ability to observe them allows for further investigation and necessary remedial action to be carried out. Also, this acquisition mode can help to track the clean-up efficiency of the methodology employed.
Minimizing matrix effects allows higher confidence in the quality of analytical data obtained. Reducing matrix concentration injected onto the analytical system is a simple and effective means to do this. When using a standard flow ESI source this can be achieved by reducing the amount of sample to be extracted, reducing the number of sample enrichment steps, or diluting final extracts. In any case, this is only a possibility if enough sensitivity is available to maintain detection at the required concentrations.
Ginger samples showed the highest ionizable background when compared to all other samples, despite having a relatively low matrix concentration (0.1 g/mL), as shown in Figure 3. Matrix effects were observed in the ginger samples with ion suppression and chromatography problems most apparent.
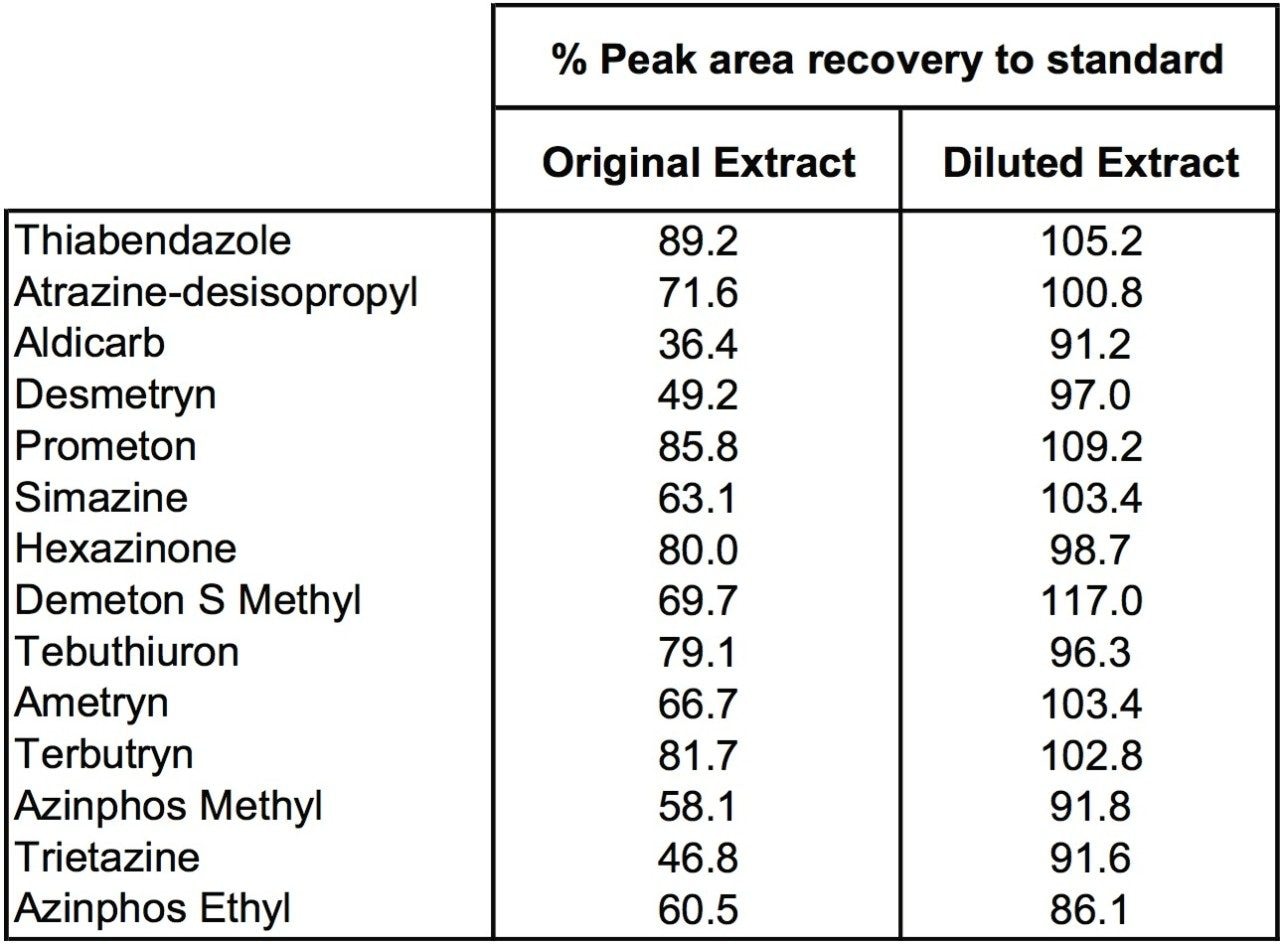
Diluting the ginger extracts 10x allowed recovery of distorted peak shape for cyromazine and reduction in matrix suppression for a number of pesticides, as shown in Figure 5. Table 1 shows reduction of ion suppression with a 10x dilution of sample. This reduction in suppression is clear when comparing peak area of pesticides in ginger to standards with no matrix present. As the matrix concentration is reduced the peak area response begins to correlate closely with standard peak areas.
720003627, July 2010