This is an Application Brief and does not contain a detailed Experimental section.
Ion mobility (IM) combined with mass spectrometry can provide information on the physical size and shape of gas-phase ions, which can assist in the optimization of synthesis of substrates to fit specific targets, and to aid the drug design process. Through the use of ion mobility and the rapid calculation of a collision cross-section (Ω), it may be possible to decide which complexes or ligands in a library (series) of possible agents have the potential for optimum activity by excluding complexes that may be either too flexible, rigid, or too small/big.
We demonstrate through example of low molecular weight anti-cancer drugs and intact protein anti-cancer drug complexes, that the travelling-wave measured collision cross-sections are consistent between the SYNAPT HDMS and G2 platforms.
Platinum-based (Pt) drugs are widely used to combat cancer. Cisplatin for example, can form adducts with DNA and cross links with specific bases, causing structural changes to DNA, and can lead to cell death. Cisplatin, however, is not active against all types of cancer, can have toxic side-effects, and can lead to resistance with repeated administration. For these reasons, the use of other transition metal-based agents as potential anti-cancer agents is being actively explored. Although reactions of Pt drugs with DNA are well understood, reactions with peptides and proteins have been less widely explored and may be important for understanding their side-effects.
In this technology brief, we report on the enhanced IM separation of ruthenium-based organometallic anticancer drugs and explore the induced conformational changes in the protein ubiquitin by reaction with the anti-cancer drug cisplatin. Experiments were performed in parallel on the SYNAPT HDMS and SYNAPT G2 HDMS systems to determine whether Ω is affected by the travelling-wave ion mobility calibration routine, the increased travelling wave1 speed, amplitude, and mobility gas pressure.
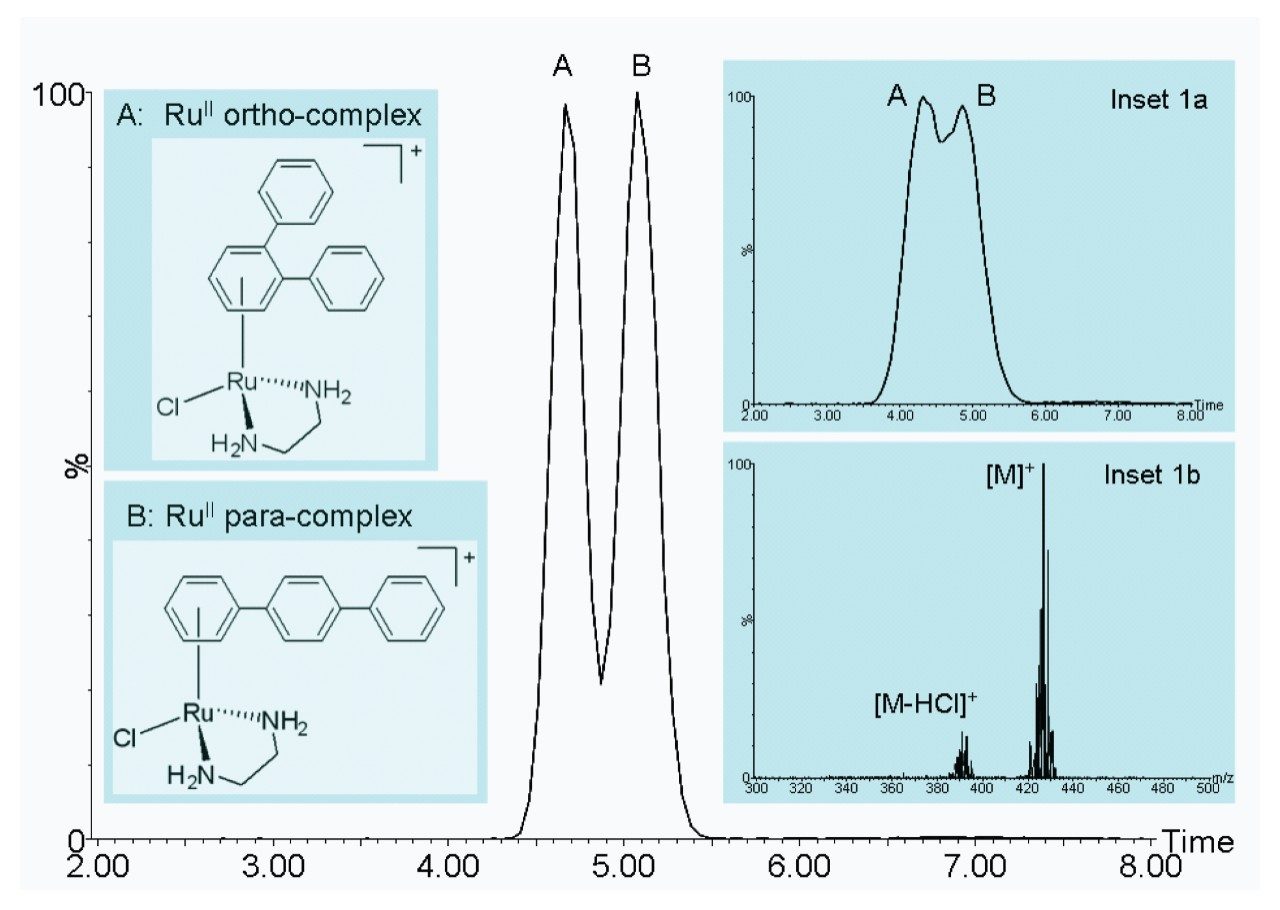
Figure 1 demonstrates the improved IM resolution afforded by the SYNAPT G2 HDMS System for the analysis of the ruthenium-based anticancer isomers. A mobility separation of ~80% valley was observed between the ortho and para-complexes. This separation was obtained using a mobility cell pressure of 3.3 mbar N2, with a travelling wave speed and amplitude of 1700 m/sec and 40 V respectively. Figure 1a shows the same compounds analyzed on the SYNAPT HDMS System. Figure 1b shows a typical mass spectrum obtained from the para-complex. The theoretically derived Ω values for the ortho and para complexes are 112.9 Å2 and 121.3 Å2 respectively. The SYNAPT HDMS T-Wave measured Ω values for the ortho and para complexes, and were 113.0 Å2 and 120.6 Å2 respectively. The SYNAPT G2 HDMS T-Wave measured Ω values for the ortho and para complexes, and were 113.3 Å2 and 119.9 Å2 respectively.
Cisplatin has been shown to have inhibitory effects on the ubiquitination process of proteins in vitro, and it is therefore relevant for understanding not only the platinum binding site(s) on ubiquitin, but also any induced conformational changes.
Figure 2 shows a comparison of the Ω values for selected dominant conformations observed for each of the multiply-charged species (7+ to 11+) obtained using both the SYNAPT HDMS and SYNAPT G2 HDMS systems for the protein ubiquitin modified with {Pt(NH3)}2+.
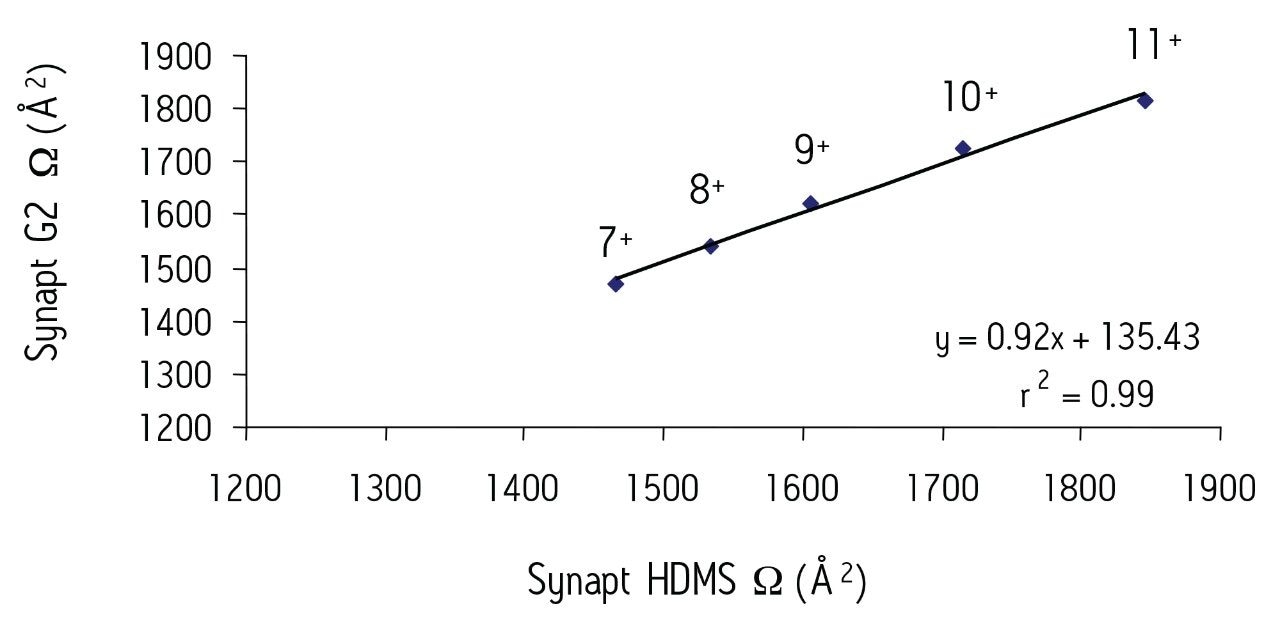
This work highlights the usefulness of the IM-MS technique for shedding new light on such protein-anticancer drug interactions. It also shows that excellent correlation between the T-Wave measured Ω values can be obtained using both variants of the SYNAPT T-Wave Technology.
Waters Corporation, MS Technologies, would like to acknowledge Prof. Peter Sadler and Dr. Abraha Habtemariam of Warwick University, United Kingdom.
720003487, June 2010