
This application note describes the increased sensitivity obtained for the analysis of two pharmaceuticals compounds, fluticasone propionate and salmeterol succinate, which are inhaled medicines and are present at very low systemic levels in the circulatory system.
The new Xevo TQ-S tandem quadrupole MS system provides a significant increase in sensitivity for the analysis of low-systemic concentration pharmaceutical compounds.
Accurate quantification of pharmaceuticals in biological fluids facilitates the correct determination of the pharmacokinetics of a medicine. Low-systemic-exposure compounds such as inhaled products or those undergoing extensive metabolism require very high sensitivity assays to accurately define the elimination phase of the pharmacokinetics curves. This need challenges the sensitivity of modern LC-MS/MS instrumentation.
The Xevo TQ-S is an ultra-high-sensitivity tandem quadrupole mass spectrometer. It is equipped with StepWave optics featuring a revolutionary off-axis ion source design. This design significantly increases the efficiency of ion transfer from the source to the quadrupole analyzer while the off-axis ion path eliminates neutral contaminants. These two factors combine to dramatically increase the sensitivity of the LC-MS/MS system.
In this application note, we describe the increased sensitivity obtained for the analysis of two pharmaceuticals compounds, fluticasone propionate and salmeterol succinate, which are inhaled medicines and are present at very low systemic levels in the circulatory system. The high data capture rate of Xevo TQ-S allows the rapid collection of numerous multiple reaction monitoring (MRM) transitions as well as simultaneous full-scan/MRM data acquisition.
|
LC system: |
ACQUITY UPLC (binary solvent manager, sample manager, HT column oven) |
|
LC column: |
ACQUITY UPLC BEH C18, 1.7 μm, 2.1 x 50 mm |
|
Gradient: |
A: 0.1% NH4OH (Aq) B: Methanol 5 to 95% B over 1.5 min |
|
Flow rate: |
600 μL/min |
|
Injection vol.: |
10 μL |
|
MS systems: |
Xevo TQ-S and Xevo TQ operated in electrospray positive mode |
|
MRM data acquisition: |
501 => 292.95 fluticasone 416.15 => 380 salmeterol |
|
Voltages: |
Capillary, cone, and collision voltage where optimized for each mass spectrometer as well as cone gas flow |
|
Source temp.: |
140 °C |
|
Desolvastion temp.: |
625 °C |
|
Nebuliser gas flow: |
1200L/Hr |
MassLynx 4.1
Quantification using TargetLynx Application Manager
The accurate quantification of pharmaceutical compounds in biological fluids is dependent upon the discrimination of the peaks of interest from the background noise. The StepWave source in the Xevo TQ-S is specifically designed to optimize this task. The source consists of two ion transfer stages which are differentially pumped; both stages are T-Wave-enabled1, stacked-ring RF devices. As the ion beam passes through the source sampling orifice it undergoes expansion. The entrance of the StepWave is designed to be large enough to efficiently capture all of the ions in this expanded ion cloud. The design of the first stage ensures that all the ions are efficiently focused and directed up into the second stage. The off-axis design ensures that any neutral materials entering the source sampling orifice are actively extracted from the system, as shown in Figure 2.
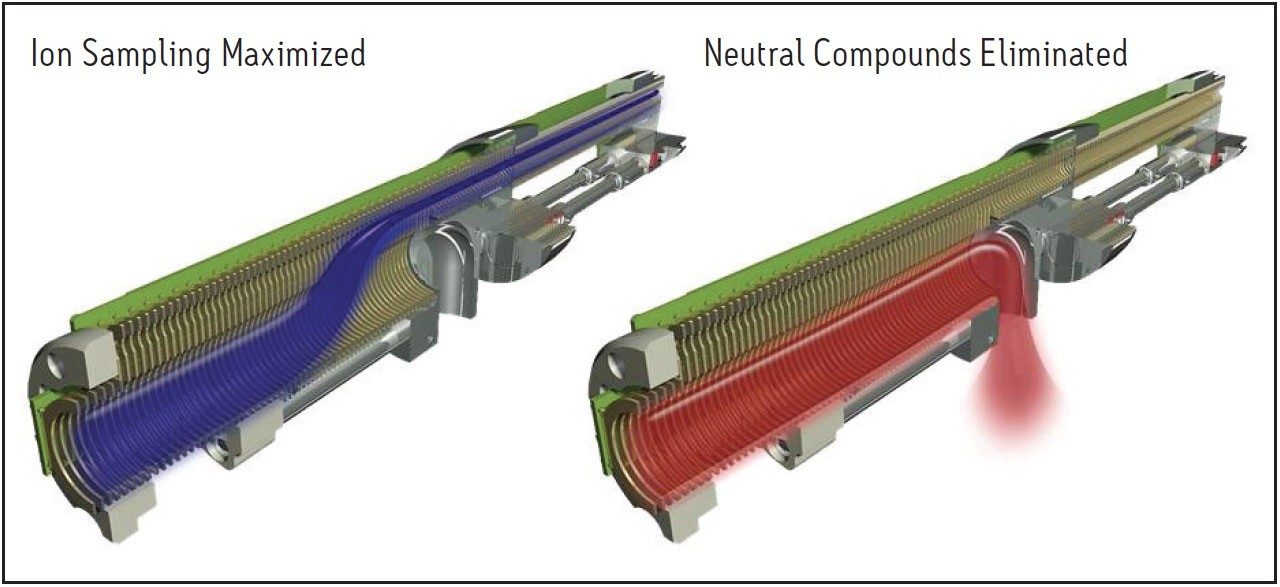
In Figure 2, we can see how the ion cloud expands as it enters the MS transfer region and then is refocused into a narrow beam as it moves onto the quadrupole analyzer region. The neutral compounds, not being charged, are unable to traverse this off-axis path and are exhausted to waste. This results in a significant improvement in the limit of detection, allowing bioanalysts to quantify analytes in plasma, serum, and urine at levels previously unobtainable.
With this StepWave design, we observed gains in sensitivity in the order of 10- to 25-fold, depending on the compound being analyzed. For example, modern medicines to treat asthma and rhinitis, such as beta 2 agonists and steroids, are designed not to enter the circulatory system and are often dosed via the inhaled route. These pharmaceutical compounds are, by design, present at extremely low levels in the systemic system, and thus are ideal for analysis with the Xevo TQ-S System.
Fluticasone propionate and salmeterol succinate were dissolved in methanol and then spiked at various physiologically-relevant levels into plasma. The plasma samples were then precipitated with acetonitrile 2:1, centrifuged, and the supernatant solution injected onto the chromatography system.
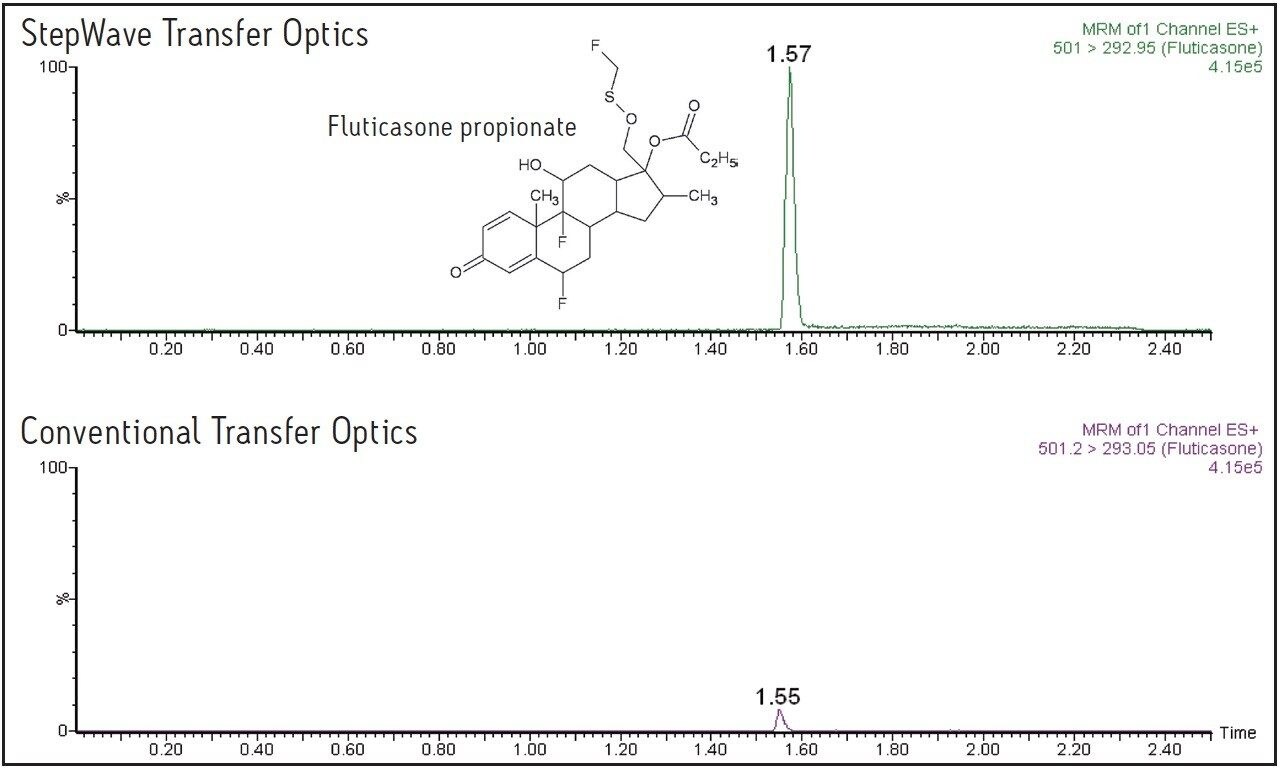
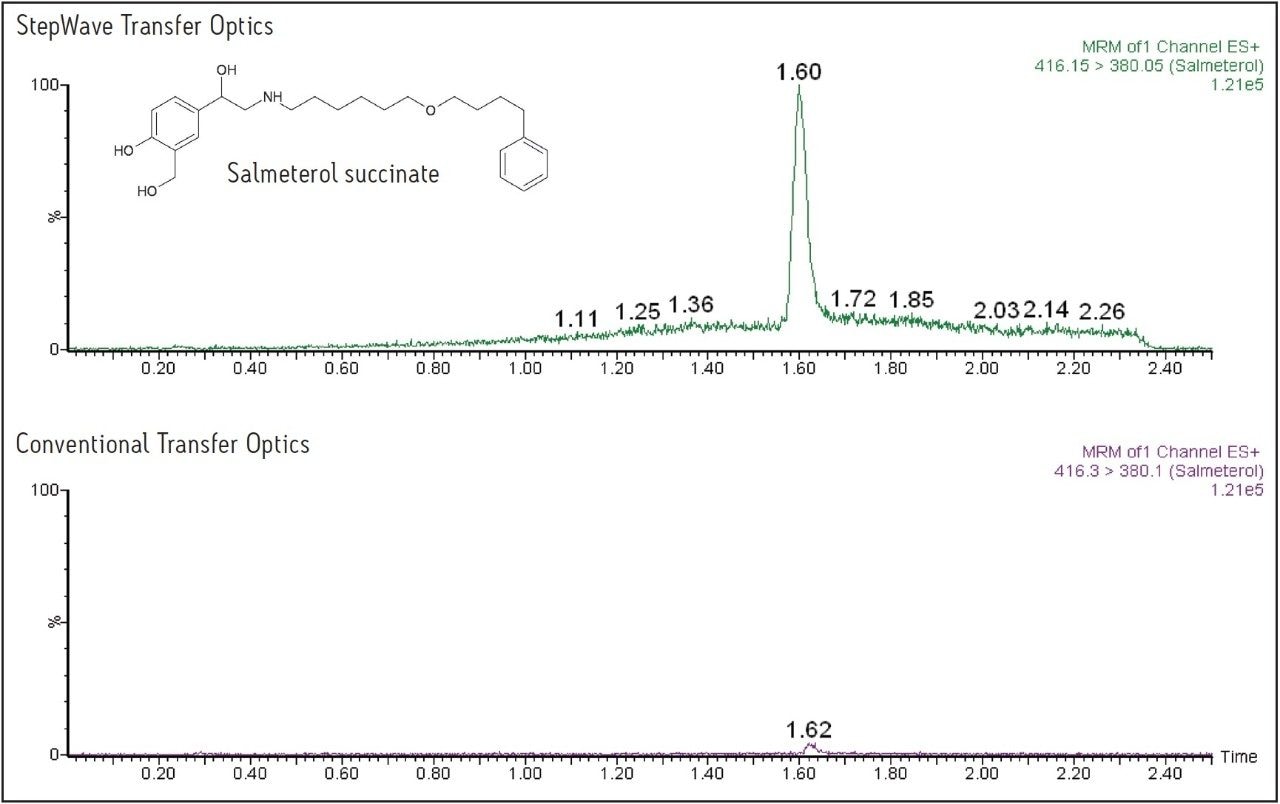
The data displayed in Figure 1 show the sensitivity increase obtained using the Xevo TQ-S for fluticasone propionate, and the data in Figure 3 show the increased response obtained for salmeterol succinate. For fluticasone propionate, the peak height was increased by a factor of 12, and for salmeterol succinate the increase in peak response was 15-fold.
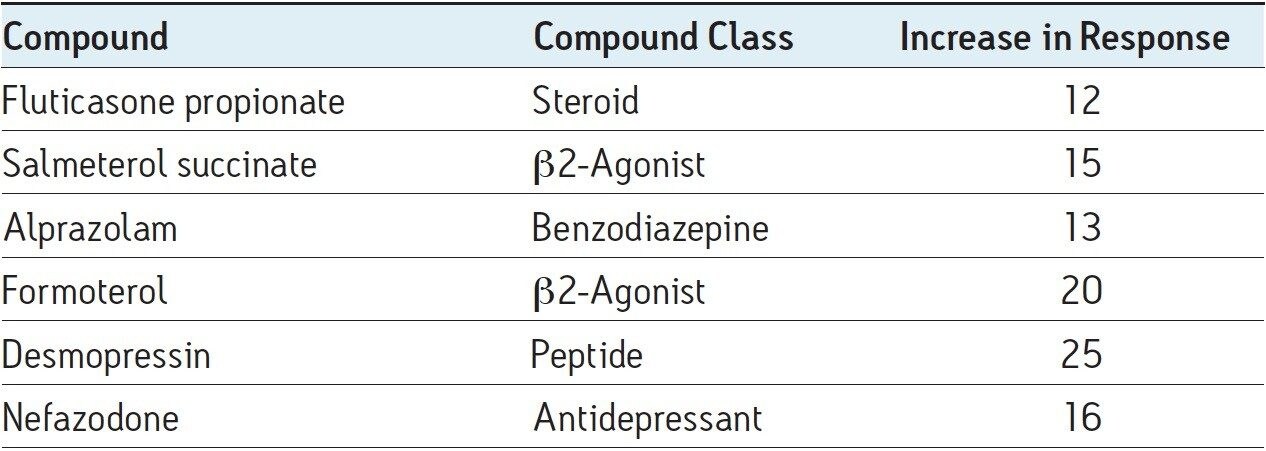
The overall increase in peak response for these two pharmaceutical compounds and other model pharmaceuticals is given in Table 1. Here we can see that the increase in peak response ranged from 12- to 25-fold.
The increased peak sensitivity has other benefits beyond lower levels of sensitivity. The greater peak response will allow faster method development, as the desired detection limits will be obtainable without the need for complex method development. The increased peak height will also make peak integration simpler, and assays more robust in general use.
The StepWave transfer optics in the Xevo TQ-S significantly increase the efficiency of ion sampling in the source. Its novel off-axis geometry design prevents unwanted, neutral compounds entering the analyzer stage of the instrument. This new design resulted in a significant increase in sensitivity, from 10- to 25-fold. This increase in assay sensitivity will allow:
720003419, May 2010