
To develop a simple UPLC-MS/MS method for the simultaneous quantitation of multiple psychotherapeutic drugs in human serum and assess its utility with authentic samples. The new method should improve upon the total analysis time, thereby increasing sample throughput and the laboratory capacity for additional samples.
Human serum samples (n=23) used for validation were obtained from the Medical Toxicology Unit where they were analysed using an established validated HPLC/UV method which involves a liquid/liquid extraction followed by a 20 minute chromatographic run.
A simple protein precipitation step was undertaken which comprised the addition of acetonitrile (300 μL) containing 3 deuterated internal standards (Clomipramine-D3, Doxepin-D3 + Imipramine-D3) to the human serum samples (100 μL). The samples were then vortex mixed for 30 seconds before centrifugation at 12000 rpm (~12000 x g) for 10 minutes. The supernatant (200 μL) was added to 5mM Acetic acid, pH 4 (200 μL) and vortex mixed for 30 seconds.
|
UPLC: |
ACQUITY UPLC System(Figure 1) |
|
Column: |
Waters ACQUITY UPLC BEH C18(2.1 x 100 mm, 1.7 μm) |
|
Column temp: |
40 °C |
|
Mobile phase: |
A=5mM Ammonium acetate + 0.05% Formic acid, B=Acetonitrile |
|
Flow rate: |
0.35 mL/min |
|
Injection vol: |
10 μL |
|
Data processing: |
MassLynx v4.1 with TargetLynx Application Manager |
|
Mass spectrometer: |
Quattro Premier XE (Figure 1) |
|
Ionisation mode: |
Electrospray positive |
|
Capillary voltage: |
3kV |
|
Collision gas pressure: |
Argon at 3.8 x 10-3 mBar |
|
Time(min) |
%B |
Curve |
|---|---|---|
|
0.0 |
10 |
Initial |
|
0.5 |
35 |
11 |
|
2.5 |
35 |
6 |
|
4.0 |
40 |
6 |
|
5.0 |
100 |
1 |
|
8.0 |
10 |
1 |
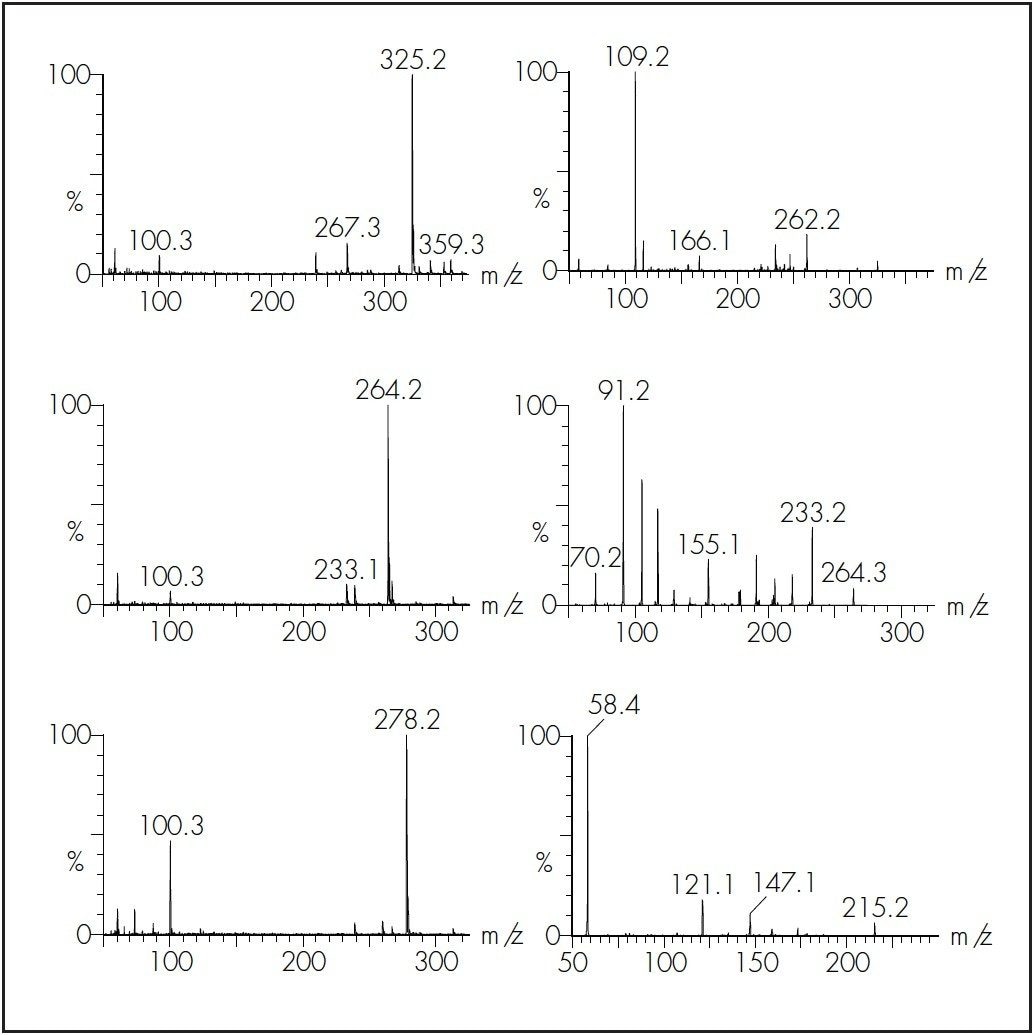
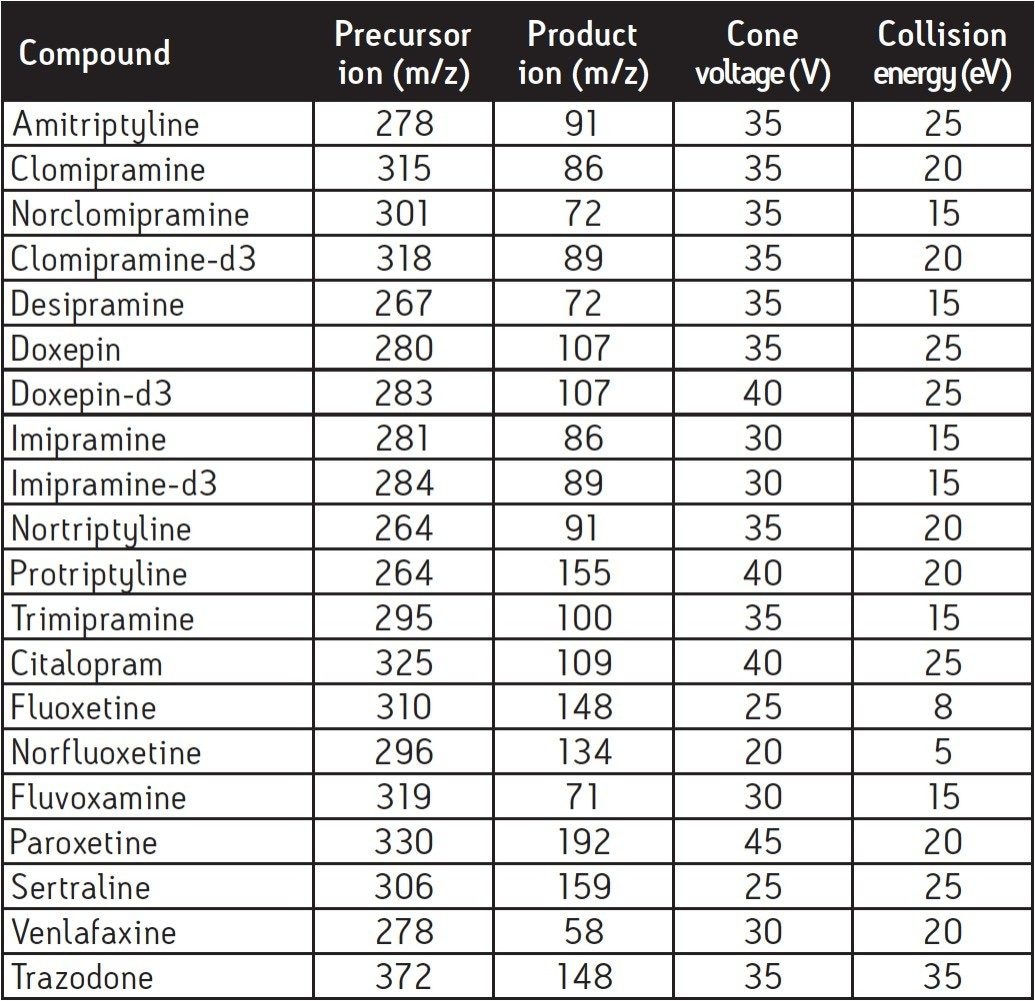
Figure 2 shows the precursor and product ion spectra for a selection of psychotherapeutic compounds. The MRM conditions used for the measurement of the psychotherapeutic drugs, metabolites and internal standards are summarised in Table 1. A quantifier and qualifier ion was monitored for each compound.
A series of calibrators (1, 2, 5, 10, 50, 100 and 200 μg/L) were prepared by adding the psychotherapeutic drugs to drug-free serum. The drugs were isolated from the matrix by acetonitrile precipitation which incorporated the internal standard addition.
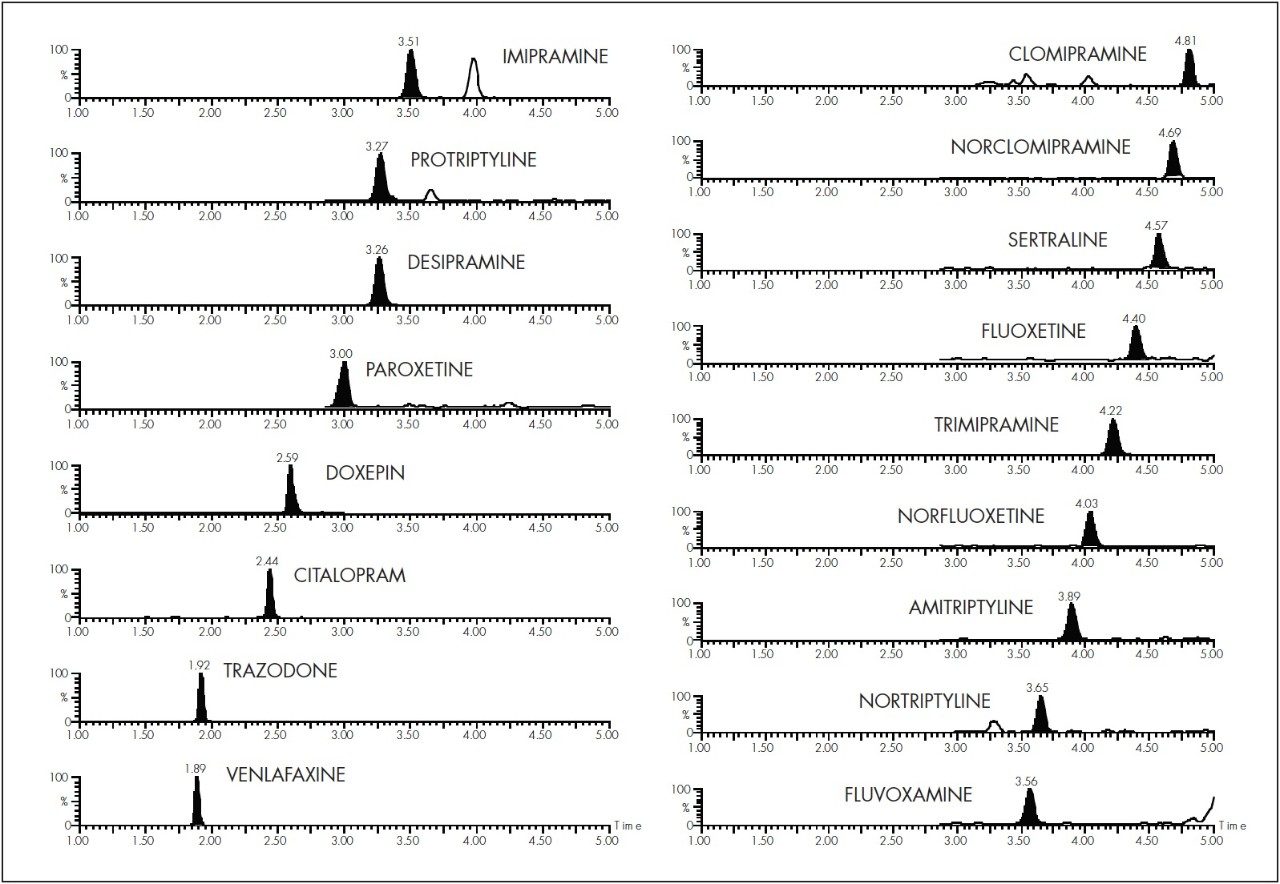
Figure 3 shows the MRM chromatograms obtained from a 10 μL injection of a 2 μg/L serum calibrator.
Quantitation was performed by the integration of the area under the peak within the specific MRM chromatogram. Figure 4 shows a typical standard curve for venlafaxine in serum. Responses were linear for all compounds, over the investigated range (coefficient of determination > r2 = 0.996).

The limits of detection were assessed for all compounds and found to range between 0.1 - 1.0 μg/L, which is below the limits required for this analysis. Figure 5 shows a patient sample containing citalopram at the lower therapeutic range.

Intra-assay precision and accuracy was assessed by adding the psycho-therapeutic compounds to drug-free serum (n=4) at a low, medium and high concentration (10, 50 and 150 μg/L). The spiked QC samples were extracted as previously described. Intra-assay precision and accuracy results were good with CV’s < 15% and > 88%, respectively. The use of protein precipitation was demonstrated to be very efficient and gave reproducible extraction recoveries > 93% for all analytes.
Matrix effects were assessed by the comparison of six different patient serum samples spiked with the psychotherapeutic compounds after extraction against the equivalent concentration solvent standards. Matrix effects were found to be acceptable with the norclomipramine response being most affected (+10%).
Addition of the 5mM acetic acid, pH 4 to the sample in the preparation phase was found to increase the stability of all compounds when assessed over 12 hours by the hourly injection of a 50 μg/L serum calibrator. It was found that was no significant change in response for any compound over the investigated time period.
As part of the testing, patient samples (n=23) were analysed by the newly developed UPLC-MS/MS method and the established HPLC/UV method, the results showed good agreement.
As the use of psychotherapeutic drugs increases the need for their analysis whether it be for therapeutic drug monitoring, clinical or forensic reasons will also grow. Therefore, there is a need for a simple, rapid analytical procedure for the analysis of these drugs.
The developed methodology has been shown to be accurate and precise in the screening and quantitation of psychotherapeutic drugs in a single 8 minute chromatographic run.
This method has been applied successfully to the analysis of clinical samples and the results compared against an established HPLC/UV method.
The objective to reduce the total analysis time was achieved through a combination of a significantly faster sample preparation by the use of protein precipitation and the use of UPLC, which more than halved the chromatographic run time. The new UPLC-MS/MS method resulted in greater laboratory efficiency and therefore sample capacity.
This application is an example of a feasible assay which can be run on Waters Instruments. Complete method validation by users is needed prior to use.
720002463, May 2008