
This application note describes a technique that compares several ball point pen ink dyes (from 3 vendors) extracted from written samples to 6 dye standards (1–6). The method utilized the Waters ACQUITY UltraPerformance LC System, which can resolve the dye standards with a simple mobile phase and 1 minute gradient. Forensic analysis can require identifying ink and age of the writing on a document. The ability to quickly identify ink dyes can facilitate these analyses for pen inks on written documents.
The Waters ACQUITY UPLC System with ACQUITY UPLC Column Technology provides a sensitive, baseline resolved separation of several triarylmethane dyes with very similar structures in less than one minute
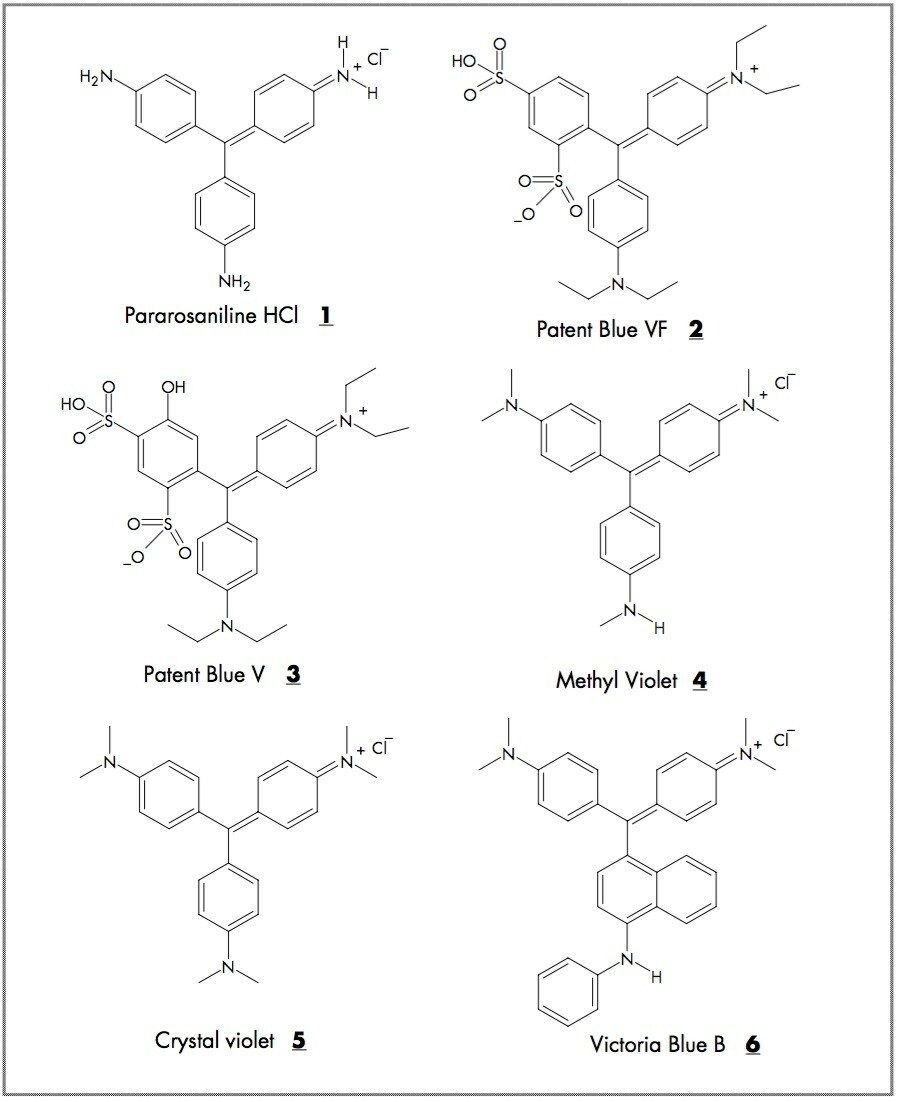
Triarylmethanedyes are used in a wide variety of applications within the chemical, pharmaceutical, and life science industries. Some industrial uses of the triarylmethanedyes (1 to 6) shown in Figure 1 are as staining agents,1,2 ink dyes,3,4 and pH indicators. These dyes are also used in medicinal applications as photochemotherapy agents,5 and binding agents (for receptors such as nicotinic acetylcholine).6 Patent blue VF (2) and V (3) injection solutions are currently used as contrast agents for visualizing lymphatic vessels.7,8
Chromatographic separation is the most widely used technique for analysis of dyes.9 The separation and analysis of the dyes from their synthesis by-products, or in product formulations can be a difficult and time consuming task.9 The typical HPLC run time is approximately 20 to 30 minutes with multiple solvent compositions and gradient steps needed to resolve the similar chemical structures. 3,4,10
This application note describes a technique that compares several ball point pen ink dyes (from 3 vendors) extracted from written samples to 6 dye standards (1–6). The method utilized the Waters ACQUITY UltraPerformance LC System, which can resolve the dye standards with a simple mobile phase and 1 minute gradient. Forensic analysis can require identifying ink and age of the writing on a document. The ability to quickly identify ink dyes can facilitate these analyses for pen inks on written documents.
The Waters ACQUITY UPLC System consisted of the ACQUITY UPLC Binary Solvent Manager, the ACQUITY UPLC Sample Manager (20 μL loop) and the Waters 2996 Photodiode Array Detector with a low volume flow cell. Waters Empower Chromatography Data System was used to control, collect and analyze all data.
Separations were performed on a 2.1 x 50 mm ACQUITY UPLC BEH C18 Column (1860002350) with 1.7 μm particle size at a flow rate of 1.0 mL/minute. Column temperature was set to 50 ºC and injection volumes for all samples and standards were set to 10 μL. 5% CH3CN in H2O was used as the weak wash solvent (500 μL) and 50/50v% CH3CN/H2O (50 μL) as the strong wash solvent.

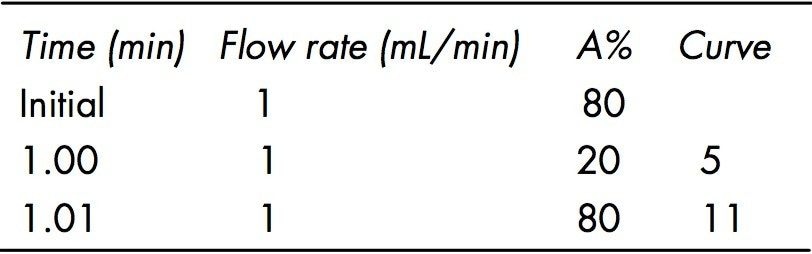
The injection method was a partial loop injection with 4 μL air gaps at pre-and post-aspiration. The seal wash solution was 10% CH3CN in H2O and the seal wash was set to 5.0 minutes. Mobile phase components and gradient conditions are in Table 1. The PDA data acquisition range was 280 nm to 700 nm using a sampling rate of 20.0 points per second, resolution 1.2, and a filtering constant of 0.1 seconds. Auto exposure, interpolate at 656nm and digital filter were enabled. Chromatograms of the standard components were extracted at UV λmax for each component.
Dyes: Pararosaniline (1), Patent Blue VF (2), Patent Blue V (3); Crystal Violet (5), and Victoria Blue B (6) were purchased from Sigma-Aldrich. Methyl Violet (4) is a known impurity of 5.
Dyes 1 to 6 were dissolved and diluted with a solution of 70v% of 1.25% acetic acid with 30v% of CH3CN to make a set of dye standard solutions (0.25 to 5 μg/mL) which were placed in screw cap 12 x 32 mm UPLC max recovery sample vials (186000327C) for analysis.
Ink extracts: 10 lines, each 1 cm long, were written with each ballpoint pen on a 20lb XEROX multipurpose 4200 paper. The sample with writing was cut (0.5 x 1 cm2) and placed in a screw cap UPLC sample vial with 500 μL to which solvent (1.25% acetic acid:CH3CN = 70:30 v/v%) was added. The vial was gently rotated with a Clay Adams NutatorMixer for one hour. The solution was transferred to a screw cap UPLC max recovery sample vial for analysis.
The six triarylmethanedye standards mixtures (1to 6) from 0.25 μg/mL to 5 μg/mL are easily separated in less than one minute even though the structures are very similar. The chromatogram shown in Figure 2 is a timed wavelength chromatogram extracted at the UV λmax of each component: 540 nm (1), 636 nm (2, 3), 585 nm (4, 5) and 615 nm (6).
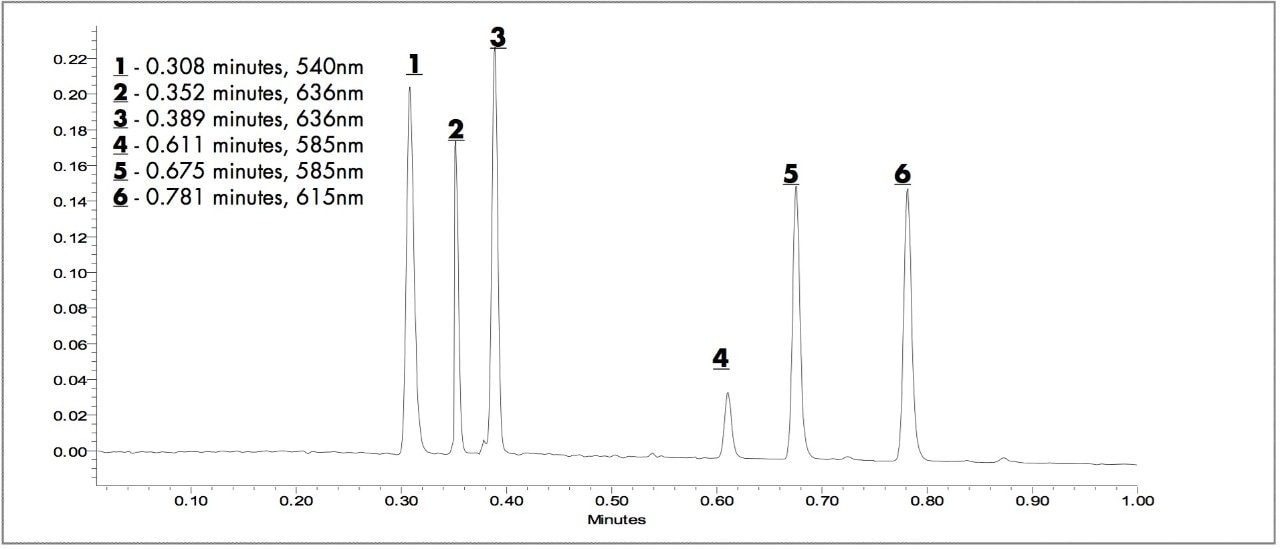
The ACQUITY UPLC System provides a 20 to 30 fold decrease in run time relative to HPLC for these dyes. The elution order is 1, 2, 3, 4, 5, and 6and the limit of detection is estimated at <0.05 μg/ mL. The extracts of writing samples using blue ball point pens from vendors B and S, and black ball point pens from B, S, and Z were analyzed using the same conditions as the standards.
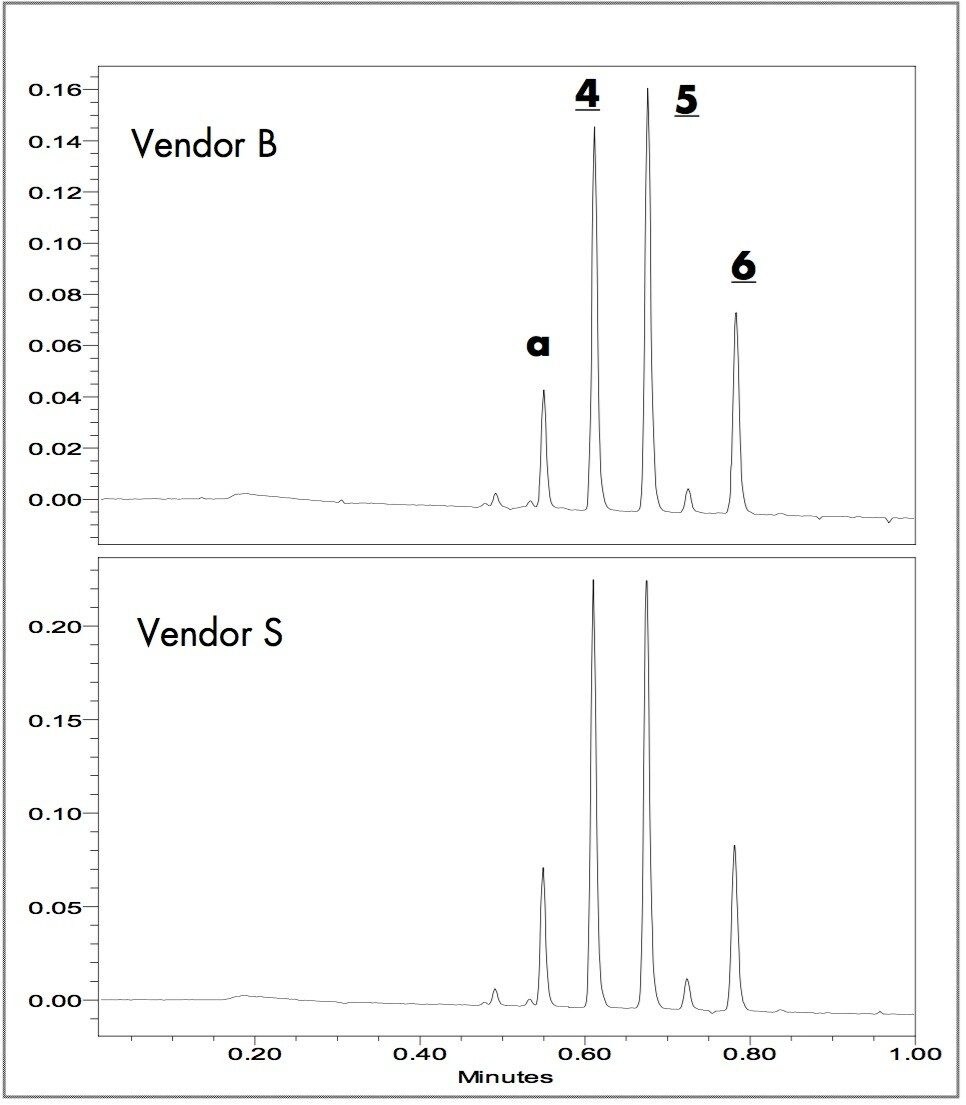
The timed wavelength extracted chromatograms of the blue writing samples at 574, 585 and 615 nm are in Figure 3. Both brands of blue ink pens contain Methyl Violet (4), Crystal Violet (5) and Victoria Blue B (6). Although the formulations contain the same components, there are differences in the relative peak ratios that might be used to differentiate each brand. Component awith UV λmax of 574 nm was not one of the standard dyes and was not identified.
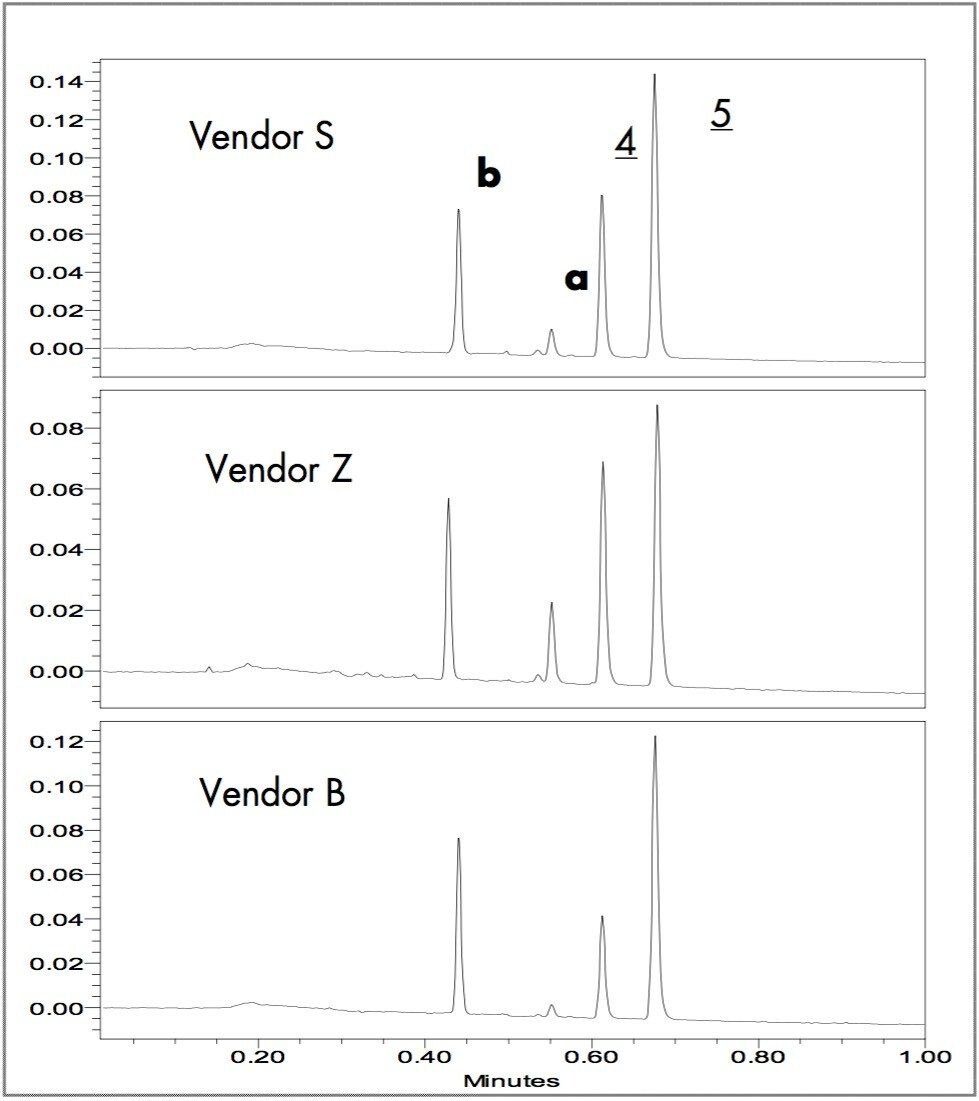
The extracted timed wavelength chromatograms of the black writing samples are in Figure 4. The black writing extracts have four components; two match Methyl Violet (4) and Crystal Violet (5). The other components [b(λmax of 420 nm) & a] were not in the standard mixture; a MS detector could provide additional information. The relative peak intensity differences have a high potential for use in forensic analysis to differentiate and identify the black pen used to write a document.
The Waters ACQUITY UPLC System with ACQUITY UPLC Column Technology provides a sensitive, baseline resolved separation of several triarylmethane dyes with very similar structures in less than one minute. This is 20–30 times faster than conventional HPLC systems for analyzing ball point pen writing extracts. ACQUITY UPLC can differentiate the written extracts from different pens with UV/PDA detection.
720001262, June 2005