
This application note describes a rapid method for the determination of a range of acidic herbicides in water samples using large volume direct injection by LC-MS/MS using the ACQUITY UPLC I-Class System coupled to the Xevo TQ-XS to achieve best degree of performance and ultra-high sensitivity.
Specific, targeted method for the determination of 20 acidic herbicides in water samples that is suitable for monitoring both drinking water and surface waters with minimal sample preparation, for compliance with European regulatory limits.
The removal of weeds from agricultural crops is an important contribution to maintaining the productivity and the quality of those crops. Herbicides are a specific group of plant protection products used to treat a variety of weeds integrated with other control practices such as crop rotation, tillage, and fallow systems. Acidic herbicides comprise families of compounds that include derivatives of benzoic acid (e.g. dicamba), acetic acid (e.g. 2,4-dichlorophenoxyacetic acid [2,4-D]), propanoic acid (e.g. fluazifop), butanoic acid (e.g. 4-(2,4-dichlorophenoxy)butanoic acid [2,4-DB]), picolinic acid (e.g. clopyralid), and other miscellaneous acids such as thiadiazine dioxide (bentazone), and imidazolinones (e.g. imazapyr). Some of the acidic herbicides have been commercially available for almost 80 years and are widely approved for use in agriculture and recreational areas such as golf courses and water courses worldwide. Herbicides can enter water bodies either directly through spray or spray drift, or indirectly via surface water runoff or leaching and sub-surface draining. In the aqueous environment, residues of phenoxyacetic acid herbicides are most commonly found in the form of the free acid.
The presence of pesticides in water is monitored globally, modelled after international norms from the World Health Organization (WHO) which are typically used as the basis for regulations worldwide.1 Presence of pesticides in European waters is regulated through different directives. The EU Drinking Water Directive sets a maximum limit of 0.1 μg/L for individual pesticide residues present in a sample (0.5 μg/L for total pesticides).2 The Water Framework Directive (WFD) deals with surface waters, coastal waters, and groundwater.3 Member States must identify River Basin Specific Pollutants and set their own national environmental quality standards (EQSs) for substances that may have a harmful effect on biological quality and have been identified as being discharged into the water environment in significant quantities. Values for these EQS vary across Europe; for example, the annual average EQS for 2,4-dichlorophenoxyacetic acid (2,4-D) was reported as 0.1 μg/L in France and Germany but 26 μg/L in the Netherlands.4 In the USA, drinking water is regulated under the Safe Drinking Water Act, where there are varying maximum contaminate levels for each residue.5
Other environmental waters are regulated under the Clean Water Act.6 Although regulations vary from country to country, many look to guidelines established by the WHO, EU, or USA.
There is a need for reliable analytical methods for monitoring acidic herbicides in drinking and surface waters. Historically used methods are liquid-liquid extraction or solid-phase extraction (SPE) as a concentration step. Liquid chromatography with tandem mass spectrometry (LC-MS/MS) has now supplanted gas chromatography-mass spectrometry (GC-MS) due to ease of use and no need or minimal sample preparation.
This application note describes a rapid method for the determination of a range of acidic herbicides in water samples using large volume direct injection by LC-MS/MS on Waters ACQUITY UPLC I-Class System coupled to the Xevo TQ-XS to achieve best degree of performance and ultra-high sensitivity.
Aliquots of surface water samples (10 mL) were centrifuged and passed through a syringe PVDF filter (0.2 μm). Aliquots (1.5 mL) from each water sample were then transferred to deactivated glass vials and acidified (30 μL of 5% formic acid) prior to analysis. The accuracy (trueness and precision) of the method was assessed by analysis of the water samples. Two different samples of drinking (DW) and surface waters (SW), previously shown to be blank, were spiked with the compounds of interest at 0.02 and 0.1 μg/L three times; n=6 for each water type at each concentration. Solutions of standards were prepared over the range 0.01 to 1.0 μg/L in UPLC water, drinking and surface water, to evaluate linearity of response and to determine the concentration of analytes in the spikes (using bracketed calibration).
|
UPLC system: |
ACQUITY UPLC I-Class with FTN Sample Manager equipped with a 250 μL extension loop, 500 μL sample syringe and 15 μL sample needle |
|
Column: |
ACQUITY UPLC HSS T3, 1.8 μm, 2.1 × 150 mm |
|
Mobile phase A: |
0.02% formic acid (aqueous) |
|
Mobile phase B: |
Methanol |
|
Flow rate: |
0.4 mL/min |
|
Injection volume: |
250 μL |
|
Loop offline: |
Automatic |
|
Column temp.: |
40 °C |
|
Sample temp.: |
10 °C |
|
Run time: |
15 min |
|
Time (min) |
%A |
%B |
Curve |
|---|---|---|---|
|
0.0 |
80 |
20 |
– |
|
9.0 |
0 |
100 |
6 |
|
12.0 |
0 |
100 |
6 |
|
15.0 |
80 |
20 |
1 |
|
MS system: |
Xevo TQ-XS |
|
Ionization: |
ESI +/- |
|
Capillary voltage: |
+2.0/-1.0 kV |
|
Desolvation temp.: |
300 °C |
|
Desolvation gas flow: |
1000 L/Hr |
|
Source temp.: |
120 °C |
|
Cone gas flow: |
150 L/Hr |
|
Collision gas flow: |
0.14 mL/min |
|
Nebulizer gas pressure: |
7 Bar |
The data was acquired using MassLynx MS Software v. 4.2, and processed using TargetLynx XS Application Manager. The selection of MRM transitions and optimization of critical parameters was performed by infusion of individual solutions of all the analytes and evaluation of the data by IntelliStart Software to automatically create acquisition and processing methods. Table 1 summarizes conditions for all MRM transitions including the retention times. Soft ionization mode was enabled for MCPB, dicamba, 2,4-DB and triclopyr.

Some of the compounds of interest, 2,4-DB, dicamba, MCPB and triclopyr, exhibited fragmentation within the source region under typical settings. Therefore, the temperature of the source block and the desolvation gas was reduced to 120 °C and 300 °C, respectively, which increased the response of the deprotonated molecular ion. These compounds were also acquired in soft ionization mode, a function enabled in the MS acquisition file that applies a shallower gradient of voltages to the StepWave XS ion transfer optics to reduce fragmentation during transmission of ions to the first quadrupole. Reducing fragmentation can result in significant improvements in sensitivity as shown in Figure 1.
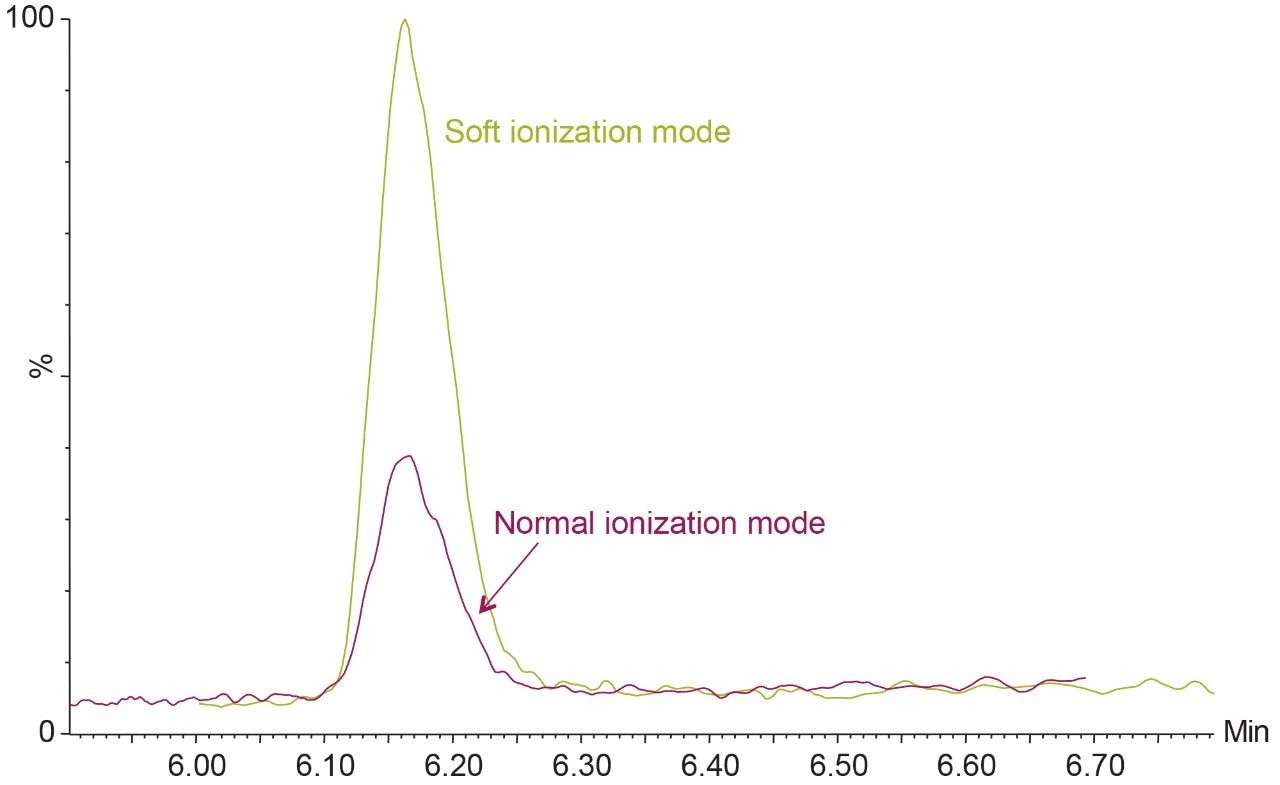
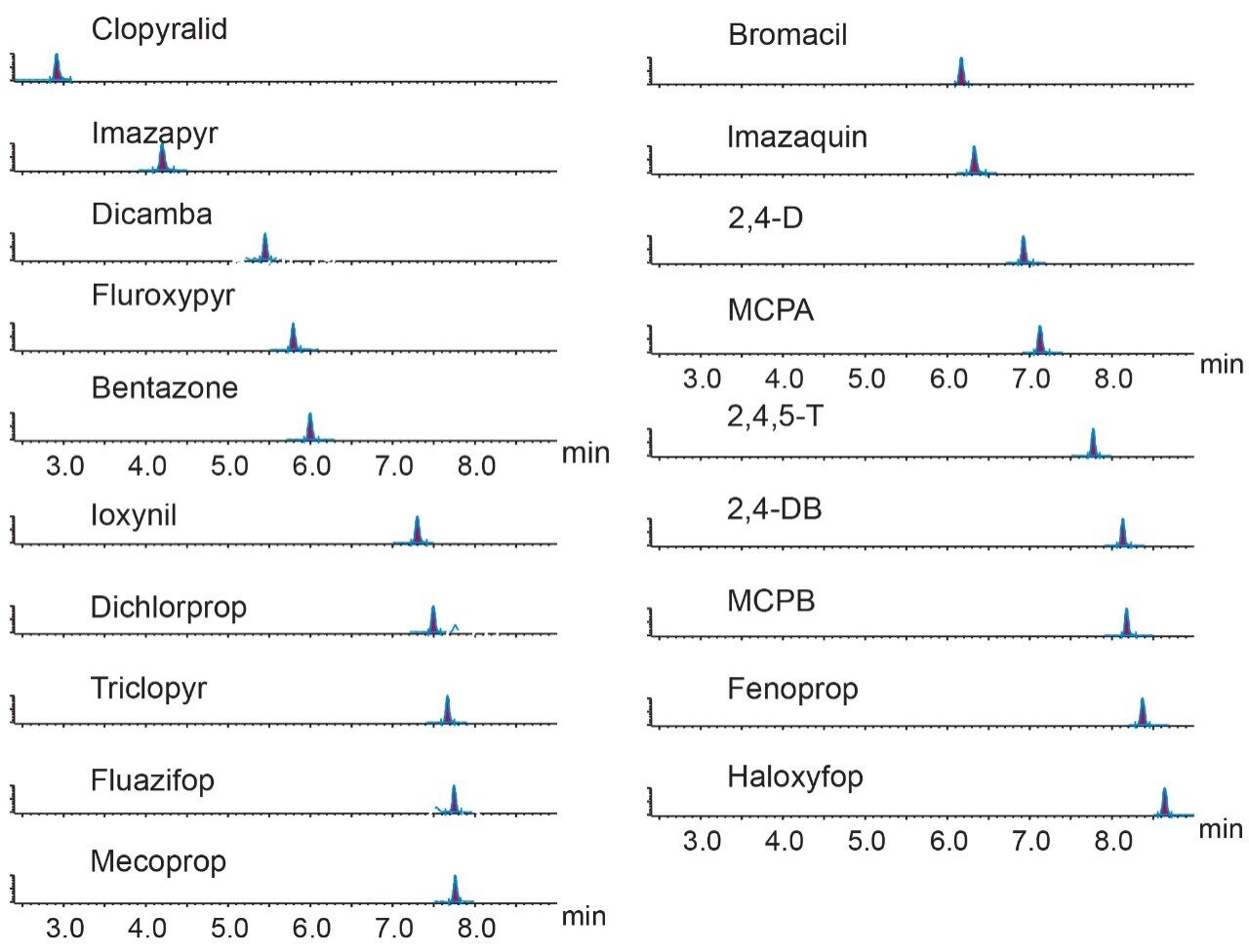
Excellent sensitivity and selectivity were demonstrated by the response for each compound detected from the analysis of drinking and surface waters spiked at 0.02 μg/L, which is well below the maximum limits. Figure 2 shows a representative example for surface water, while the drinking water provided comparable or better results for all of the herbicides. Laboratories are expected to provide methods with lower limits of quantification (LLOQ) of at least one third of the EQS. The sensitivity observed suggests that detection and quantification of all compounds at lower concentrations should be possible.
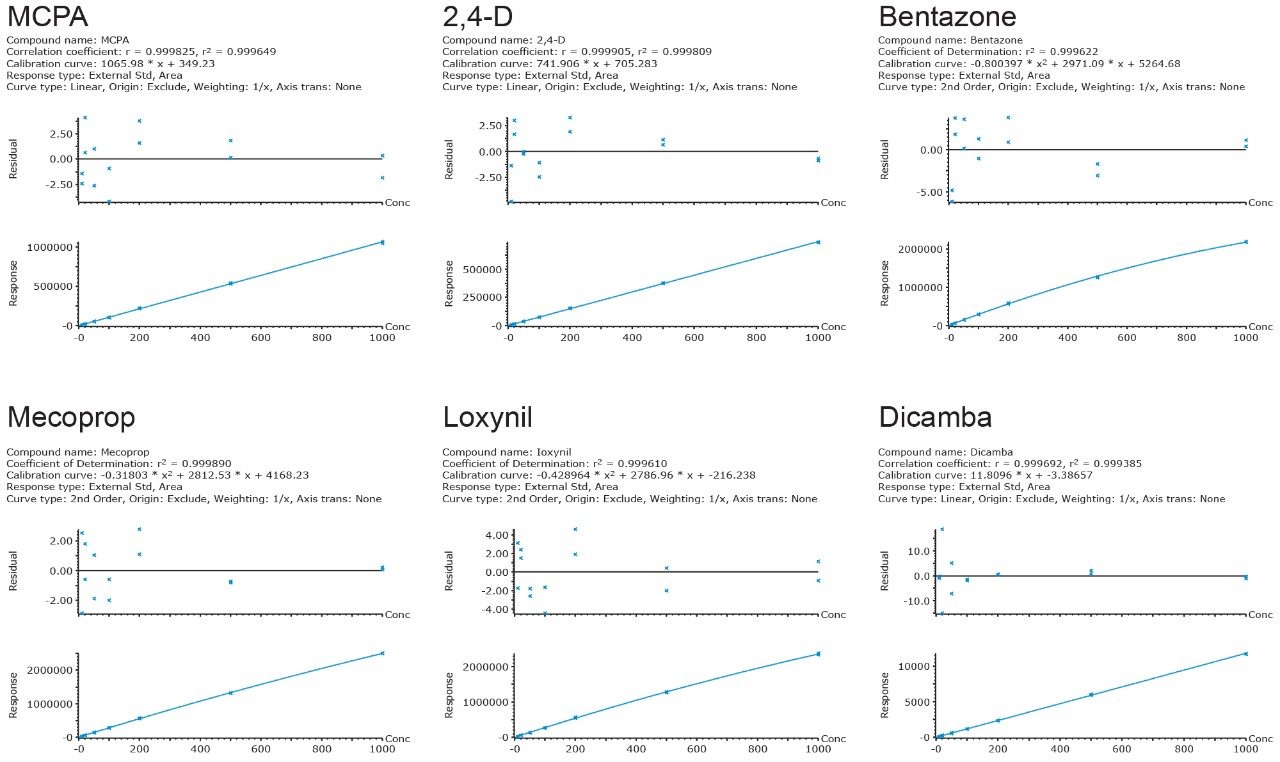
Standard solutions, prepared in drinking and surface waters at seven concentrations: 0.01, 0.02, 0.05, 0.10, 0.20, 0.50, and 1.0 μg/L, were used for calibration. The response was linear for most compounds, and where non-linearity was observed (e.g. bentazone), a second-order regression (quadratic) was applied to the compound's calibration points. In all cases, the correlation coefficients (r2) were >0.99 with residuals of <20%, as shown in Figure 3.
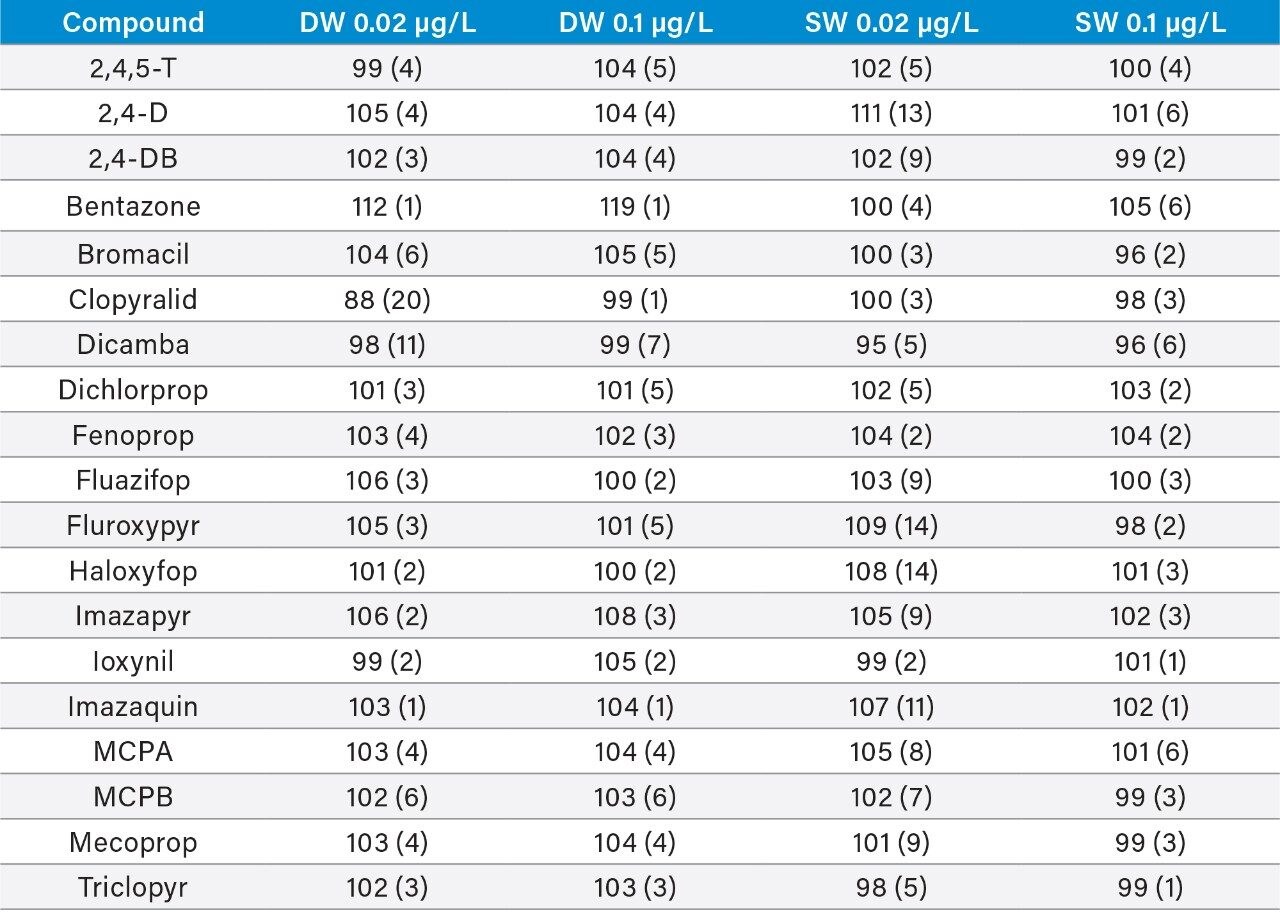
The accuracy of the method was determined from the analysis of spiked water samples. The measured values were compared with the expected values from spiking and found to be within the range 88% to 120% (Table 2). Repeatability was good with RSDs ≤7% at 0.1 μg/L and ≤20% at 0.02 μg/L.
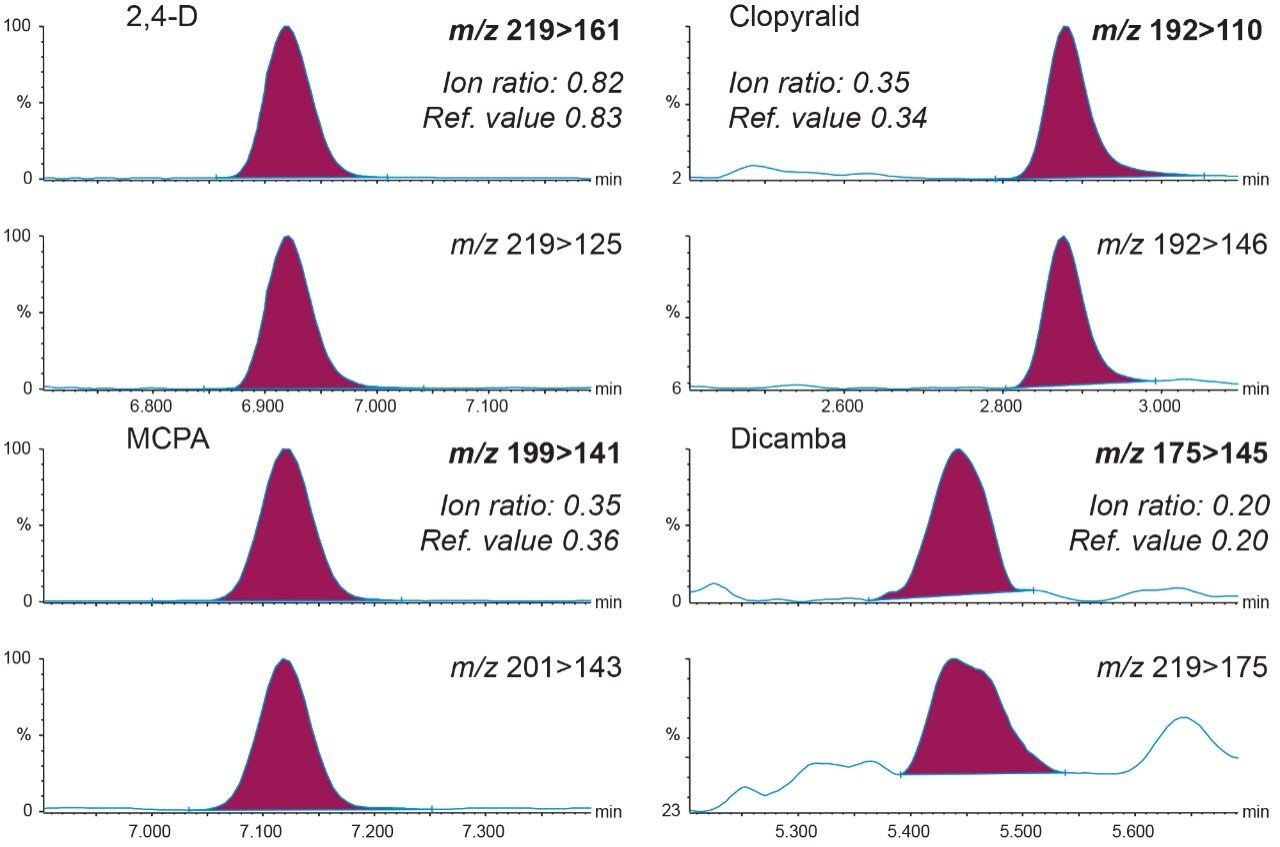
Identification criteria, ion ratios, and retention times, were all within acceptance tolerances (±30% of the reference and ±0.1 min, respectively). Figure 4 shows the transitions for a selection of acidic herbicides from analysis of surface water spiked at 0.02 μg/L.
This application note describes the performance of a method for the determination of 20 acidic herbicides by direct, large-volume injection of water samples by UPLC-MS/MS on an ACQUITY UPLC I-Class System coupled to the Xevo TQ-XS. The method is simple, fast, and reliable which avoids the time, costs, and potential losses of recovery associated with various types of sample preparation. The results of our internal validation indicate that the method is suitable for the determination of acidic herbicides in both drinking and surface waters for monitoring purposes. Calibration characteristics, linearity, and residuals were very good over the concentration range studied and accuracy of the method was shown to be excellent. Scientists must validate the method in their own laboratories and demonstrate that the performance is fit for purpose and meets the needs of the relevant analytical control assurance system.
720006307, June 2018